Dexilant
Dexilant (dexlansoprazole) belongs to a class of medicines called proton pump inhibitors (PPIs), which reduce the amount of acid the stomach produces. These drugs are often used to treat heartburn and stomach ulcers, but they may cause side effects such as serious kidney problems.
Dexilant is a modified version of Prevacid (lansoprazole). It releases medication in two stages. The first is released within an hour of taking the drug. The second release happens about four to five hours later.
Dexilant is available by prescription only, and is usually taken as one 30 mg capsule or one 60 mg capsule once daily, depending on the condition treated. There is no generic version or over-the-counter version of the drug.
Dexilant is broken down by the liver. Therefore, people with liver disease should not take more than 30 mg of Dexilant in a day.
Takeda Pharmaceuticals manufactures Dexilant. The U.S. Food and Drug Administration approved Dexilant for use in adults in January 2009. In 2016, the FDA approved Dexilant to treat certain patients from 12 through 17 years old.
What Is Dexilant Used to Treat?
Dexilant is a heartburn medication approved to treat certain conditions caused by excessive stomach acid. Other PPIs, including Prilosec, Nexium, Prevacid and Protonix, may be approved for different uses than Dexilant.
FDA Approved Uses for Dexilant
- Healing erosive esophagitis (EE; damage to the esophagus caused by stomach acid)
- Maintenance of healed EE
- Relief of heartburn with healed EE
- Treating gastroesophageal reflux disease (GERD)
Serious Dexilant Side Effects
Dexilant shares many of the same side effects as other proton pump inhibitors. This class of drugs has been on the market since the 1990s, and many studies have clarified the types of side effects that might occur with PPIs.
- Acute interstitial nephritis (AIN)
- Bone fractures
- Systemic lupus erythematosus
- Vitamin B12 deficiency
- Magnesium deficiency
- Stomach polyps
- Heart attack when taken together with certain blood thinners
- Esophageal cancer
- Stomach cancer
- Increased asthma and allergy risks for children
- Stroke
- Chronic kidney disease (CKD)
Stopping Dexilant Suddenly Can Cause Health Problems
Stopping a PPI suddenly, especially in people who have been taking one for a long time, can cause symptoms to return at a greater intensity than before. People taking Dexilant or any other proton pump inhibitor should stop only after talking with their doctor and working out a plan to safely stop.
In some cases, a doctor may be able to prescribe a proton pump inhibitor alternative. But in others, a doctor may determine that continuing Dexilant is a safer option than switching to another treatment. A 2022 study reviewed research comparing several PPIs.
Dexilant Warnings About Serious Side Effects
The Dexilant label carries several warnings and precautions about potentially serious side effects. But it does not list all potential Dexilant side effects. The FDA requires manufacturers add warnings to a drug label when there is increased scientific evidence of a possible risk.
- Stomach cancer
- Acute interstitial nephritis (AIN; damage to the tissue of the kidney)
- Severe diarrhea caused by the bacteria Clostridium difficile
- Bone fractures
- Systemic lupus erythematosus
- Vitamin B12 deficiency
- Magnesium deficiency
- Interactions with tests for neuroendocrine tumors
- Interactions with the drug methotrexate
- Stomach polyps
Dexilant does not have a boxed warning, the most serious warning the FDA can require.
People should talk with their doctor about other drugs they are taking before taking Dexilant. More than 1,800 drug products may cause proton pump inhibitor interactions if taken with Dexilant or other PPIs.
Common Dexilant Side Effects
Minor Dexilant side effects are more common than serious side effects.
- Diarrhea
- Abdominal pain
- Nausea
- Upper respiratory tract infection
- Vomiting
- Flatulence
- Headache
- Swelling of nasal passages & back of throat
- Pain in the mouth and nose
Studies Associate Dexilant with Kidney Problems
Many studies have associated long-term PPI use with serious kidney complications since the 1990s. One of these reviewed some of the largest studies of PPI use and found that PPIs can increase the risk of kidney problems. The review included 9 studies of over 2 million total people. Compared with people who did not take PPIs, those treated with PPIs had about a 30 percent to 40 percent increased risk of both short- and long-term kidney damage.
Kidney Problems Associated with Dexilant and other PPIs
- Kidney failure
- Kidney disease
- Kidney injury (acute interstitial nephritis)
Dexilant Acute Kidney Injury (AIN) Risk
Long-term use of Dexilant or other PPIs may increase the risk of a serious kidney injury called acute interstitial nephritis (AIN). AIN is defined by inflammation in the tissue of the kidney. It can often be treated if caught early, but in rare cases it can cause permanent damage.
Left untreated, AIN can lead to metabolic acidosis. That’s a condition in which the kidneys cannot remove acid from the blood. It can cause kidney failure and end-stage kidney disease.
- Blood in the urine
- Fever
- Nausea
- Vomiting
- Swelling of any part of the body
- Water retention
- Weight gain
- Fatigue
- Rash
- Mental changes (drowsiness, confusion or coma)
- Changes in urine output (either increased or decreased)
People who experience symptoms of AIN should contact their doctor immediately.
Dexilant and Kidney Disease
Studies have found that people who take PPIs may have a higher risk of serious kidney disease.

A recent review of studies evaluating the association between PPIs and kidney problems included over 2 million total people. Compared with people who did not take PPIs, those treated with PPIs had a 44 percent increased risk for acute kidney injury, a 36 percent increased risk for CKD, and a 42 percent increased risk for end-stage renal disease (ESRD; the last stage of kidney disease, where dialysis may be necessary).
One of the included studies was a 2016 study of US veterans newly treated with acid blocking drugs that was published in the Journal of the American Society of Nephrology. This study compared PPIs with another class of acid blocking drugs called H2 receptor blockers. This study found that after 5 years of treatment, PPI users were 96 percent more likely than H2 receptor blocker users to experience end-stage renal disease, and were 28 percent more likely to develop CKD.
Some studies have found that PPI-associated kidney disease might occur without patients knowing or experiencing any symptoms. Therefore, people taking PPIs may need periodic tests of their kidney function to identify these “silent” side effects of PPIs.
Although studies have found a higher risk for kidney disease in patients treated with PPIs, it is important to remember that these events are still overall not very common. For example, in the US veterans study, the increased risk for ESRD means that about 15 more patients have ESRD for every 100,000 patients treated for 1 year with a PPI instead of a H2 receptor blocker. Likewise, the increased risk for CKD means that about 1100 more patients have CKD for every 100,000 patients treated for 1 year with a PPI instead of a H2 receptor blocker. Patients taking PPIs should speak with doctor about any other conditions that might increase their risk for these side effects and whether PPIs are the best option for them.
Studies Associate Dexilant with Cancer and Heart Attack Risks
Other studies have shown long-term PPI use may increase the risk of other serious conditions, including cancers and heart attacks.
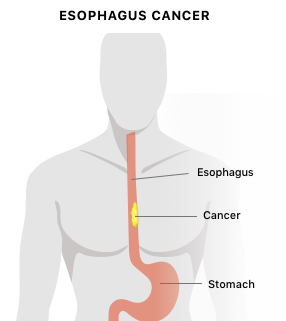
A 2018 study published in Cancer Epidemiology found PPIs may be associated with esophageal cancer. Researchers examined the medical records of 796,492 people in Sweden who took PPIs during a 7-year period. Compared with the normal rate of esophageal cancer that would be expected in people not taking PPIs, those who took PPIs experienced esophageal cancer almost 4 times more frequently than expected. However, overall, only 0.13 percent of all patients taking a PPI developed esophageal cancer. The risk of esophageal cancer was no different than expected in people taking H2 receptor blockers.
Another 2017 study from Sweden found PPIs may be associated with stomach cancer. These researchers examined data from 797,067 people who were treated with PPIs for on average 5 years. Compared with the normal rate of stomach cancer that would be expected in people not taking PPIs, those who took PPIs experienced esophageal cancer almost 3.4 times more frequently than expected. Although this is a large increase in risk, only 0.28 percent of patients taking a PPI developed gastric cancer. The risk of stomach cancer was again no different than expected in people taking H2 receptor blockers.
A 2017 study in the journal Gut also found that taking PPIs could more than double the risk of stomach cancer in people taking them for Helicobacter pylori infection, which could cause peptic ulcer disease. H. pylori infection can itself increase the risk for stomach cancer. That same year, the FDA added a gastric-malignancy warning to the labels of Dexilant and other PPIs. The warning advises people to consider screening for gastric cancer if their response to treatment with a PPI is below standard.
A 2015 study published in the journal PLOS One found that long-term PPI use was associated with an increased risk of heart attacks among patients with GERD. Another study in PLOS One found an association between PPI use and fatal heart failure among people with coronary artery disease.
Dexilant Lawsuits and Kidney Damage
People who suffered serious kidney problems after taking Dexilant have filed lawsuits over their injuries. Dexilant lawsuits are among thousands filed against manufacturers of several different brands of PPIs. The lawsuits have been combined in a multidistrict litigation (MDL) in a New Jersey federal court. MDLs allow several similar lawsuits to move more quickly through the legal process.
As of November 2019, there were 13,492 lawsuits pending in the proton pump inhibitor MDL.
People who filed PPI lawsuits claim Dexilant or other PPIs caused kidney disease, injury or other serious conditions.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.