Tasigna Side Effects
In Tasigna clinical trials, at least 20 percent of people treated with the chemotherapy drug suffered mild side effects such as diarrhea, headache, fatigue and runny nose. The drug has a black box warning for QT prolongation, which is a dangerous irregular heart rhythm that can lead to sudden death. Studies also show it may cause reduced blood flow to the legs, heart, and brain.
Tasigna (nilotinib) side effects range from nausea, diarrhea and headaches to cardiovascular problems and irregular rhythms that may lead to sudden death.
From 2007 to September 30, 2019, Americans reported 16,478 cases of adverse events related to Tasigna, according to the FDA Adverse Events Reporting System (FAERS). Of those, 11,532 were serious, including 3,100 deaths.
Lawsuits against Tasigna’s manufacturer, Novartis, claim the company failed to warn the public about a dangerous side effect called atherosclerosis. The disease occurs when fatty deposits clog arteries, and it may lead to heart attacks, strokes and other complications.
A November 2022 study published in Frontiers in Cardiac Medicine found leukemia patients treated with nilotinib had a higher risk of cardiovascular adverse events than those treated with a similar drug, imatinib.
Common Side Effects
Common side effects of Tasigna can happen immediately after taking the drug. These are typically less serious and may go away after a short time. In clinical trials, the most common side effects occurred in 20 percent or more of participants.
- Anemia
- Constipation
- Cough
- Diarrhea
- Fatigue
- Headache
- Joint pain
- Pain in the extremities
- Nausea
- Night sweats
- Pruritus (itchiness)
- Raised body temperature
- Rash
- Stuffy or runny nose
- Thrombocytopenia (low platelet count that may lead to increased bleeding and bruising)
- Vomiting
Side Effects After Stopping Treatment
Patients who achieve good blood cell counts may be eligible to stop treatment. Doctors call this treatment free remission, or TFR. However, stopping Tasigna may cause side effects.
People who try TFR may suffer more musculoskeletal problems after stopping the drug. These include pain in the muscles, arms, legs, joints, bones and spine. Patients who experience these symptoms should inform their doctor.
Atherosclerosis
Studies and other reports have linked Tasigna to atherosclerosis. The drug’s United States prescribing information does not warn specifically about this condition, but does warn about atherosclerosis-related conditions.
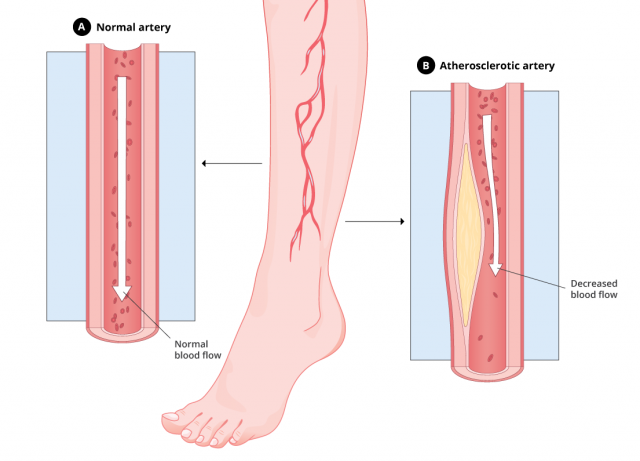
Atherosclerosis, or hardening of the arteries, happens when fatty deposits build up in the blood vessels. This decreases blood flow to other parts of the body and may lead to stroke or heart attack.
Treatment may include medications, surgeries, or diet and lifestyle changes.
Health Canada warned the public in 2013 about atherosclerosis-related conditions associated with Tasigna. A review of the Novartis global safety database revealed 277 cases of atherosclerosis reported between January 2005 and January 2013, according to the agency.
Peripheral Artery Disease: A Type of Atherosclerosis
Peripheral artery disease, or PAD, is a type of atherosclerosis that causes narrowing of the blood vessels in the legs, stomach, arms and head. PAD usually affects the legs. It can lead to pain, numbness and tissue death called gangrene.
A letter to the editor published in the peer-reviewed medical journal Leukemia in 2013 analyzed studies that had found PAD was more prevalent in people who used Tasigna versus a similar Novartis drug called Gleevec.
“The detrimental effect of nilotinib was evident despite a shorter duration of treatment,” the letter’s author Dr. A. Tefferi wrote.
Tefferi, a hematologist and internist with the Mayo Clinic, encouraged full disclosure to patients of the adverse events associated with drugs like Tasigna, including the risk of Tasigna-associated accelerated atherosclerosis.
How Long Does It Take to Develop Atherosclerosis After Taking Tasigna?
Normally, atherosclerosis develops gradually over several years, but a paper published in Leukemia said some people developed accelerated atherosclerosis after just two and a half years of treatment with Tasigna.
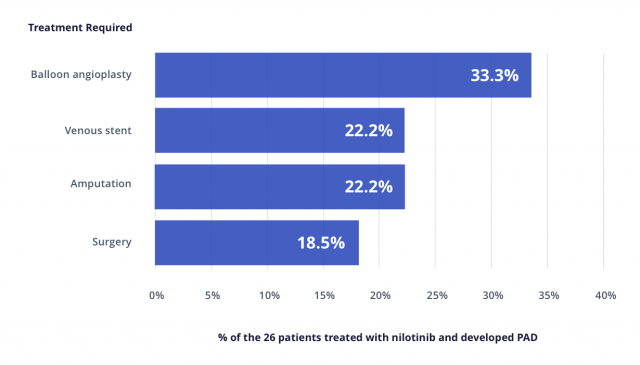
The paper published in Leukemia also mentions a study by Dr. Kim an colleagues where 129 people were screened for PAD after treatment with Tasigna or Gleevec.
Researchers found that 26 percent of people treated with Tasigna as first-line therapy and 35.7 percent of patients treated with Tasigna as second-line therapy suffered PAD versus only 6.3 percent of patients treated with Gleevec as first-line therapy.
In this study, patients developed PAD 21 to 56 months after starting Tasigna.
Depending on which arteries are blocked, atherosclerosis symptoms may affect the heart, brain, limbs or kidneys.
Myocardial Ischemia
Myocardial ischemia is another possible side effect of Tasigna. It occurs when blood flow to the heart is reduced.
Atherosclerosis affecting the coronary arteries is the most common cause of myocardial ischemia.
After lack of oxygen and blood starves the heart muscle, it begins to die. This leads to myocardial infarction, or heart attack.
Treatment for myocardial ischemia may include medication, surgery or diet and lifestyle changes.
According to the Tasigna medication insert, about 5 percent of people suffered ischemic heart disease-related cardiac events with the 300 mg dose in clinical trials. About 9.4 percent suffered the condition with the 400 mg dose.
In a 2016 study published in the Annals of Internal Medicine, researchers studied 896 patients who took Tasigna or other similar drugs. They found that the rate for myocardial infarction was highest in people who took Tasigna compared to other similar drugs.
The most common symptom of myocardial ischemia is chest pain. It can feel like chest discomfort, heaviness, tightness or heartburn. Call 911 immediately if myocardial ischemia symptoms last for more than five minutes. These symptoms could mean a heart attack is occurring.
Black Box Warning for QT Prolongation
Tasigna carries a black box warning for the risk of QT prolongation and sudden death. Long QT syndrome is a condition that occurs when the heart’s rhythm becomes irregular because of abnormalities in the heart’s electrical system. QT prolongation may lead to loss of consciousness or sudden death.
Women and the elderly are more vulnerable to drug-induced QT prolongation, according to the Sudden Arrhythmia Death Syndromes Foundation.
Some people may be born with abnormalities in their heart’s electrical system, but a number of drugs may also cause this problem. The risk of QT prolongation increases when people take Tasigna with drugs known as strong CYP3A4 inhibitors. These drugs include clarithromycin, diltiazem, erythromycin, itraconazole, ketoconazole, ritonavir and verapamil.
According to Tasigna’s website, people should undergo an electrocardiogram (ECG or EKG) to check their heart’s electrical activity before taking the drug, 7 days after starting treatment, following any dose changes and regularly throughout treatment.
Symptoms of QT Prolongation
Common symptoms of long QT syndrome range from sudden loss of consciousness to sudden death. These events may occur without warning at any time during treatment with Tasigna.
Unlike a heart attack, QT prolongation does not cause chest pain or persistent shortness of breath.
In people who lose consciousness, the heart’s rhythm may return to normal in about one minute and restore consciousness. If the rhythm fails to correct itself, the patient will need emergency medical attention to correct it.
Other Serious Side Effects
In addition to cardiovascular issues, such as QT prolongation, Tasigna’s medication guide warns of a few other serious side effects.
Low Blood Cell Counts
Tasigna may cause decreased blood flow to the legs, heart or brain. The medication guide does not explain the cause of decreased blood flow. Symptoms include: chest pain or discomfort; numbness or weakness; and change in skin color, coldness or pain affecting the leg.
Decreased Blood Flow
Tasigna may cause decreased blood flow to the legs, heart or brain. The medication guide does not explain the cause of decreased blood flow. Symptoms include: chest pain or discomfort; numbness or weakness; and change in skin color, coldness or pain affecting the leg.
Pancreas Inflammation (Pancreatitis)
Some people who took Tasigna developed pancreatitis, or inflammation of the pancreas. Tasigna may increase levels of lipase, an enzyme that helps break down fats in the blood. High levels of lipase usually indicate a problem with the pancreas.
Liver Problems
Tasigna can increase the risk of liver problems, especially in people with a history of liver problems. Symptoms of liver problems include stomach pain, yellowing of the skin or eyes, and dark-colored urine.
Tumor Lysis Syndrome
Tumor lysis syndrome may occur when cancer cells breakdown quickly after a patient takes Tasigna. It can lead to kidney failure and abnormal heartbeat. Some people may require kidney dialysis.
Bleeding
Some people who took Tasigna suffered bleeding problems. Some cases were severe and patients died. Watch for signs of bleeding, such as bruising or blood in urine and stool. Patients should inform their doctors immediately if they notice symptoms of bleeding.
Fluid Retention
Tasigna may cause your body to hold too much fluid. Symptoms of fluid retention include shortness of breath, swelling, and weight gain.
Abnormal Growth or Development
It’s possible that children who take Tasigna may suffer abnormal growth or development. The long-term effects of prolonged treatment with Tasigna in children are unknown.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


