Valsartan Recalls
On July 13, 2018, the FDA announced a voluntary recall of some valsartan products because of contamination with NDMA, a potentially cancer-causing chemical. Other drugs affected by the valsartan recall include losartan and irbesartan.
Why Was Valsartan Recalled?
Drug manufacturers issued valsartan recalls because the drug was contaminated with N-nitrosodimethylamine, or NDMA, a chemical that may cause cancer. Not all valsartan products are included in the recall. The FDA has traced the recalled drugs to Chinese manufacturers Zhejiang Huahais and Zhejiang Tianyu as well as Hetero Labs Limited in India.
According to a statement released by the FDA on August 30, 2018, the initial recall involved more than half of the United States supply of the valsartan. The agency estimated that the tainted drugs first entered the US market around 2014. In September 2018, the FDA announced it had found another toxic chemical in tainted valsartan batches called N-nitrosodiethylamine (NDEA). Similar to NDMA, the chemical could possibly cause cancer.
As of 2022, the recall now includes some lots of other drugs in the same class as valsartan called angiotensin II receptor blockers (ARB) after manufacturers found N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) in some of these drugs in March 2019. So far, in addition to the valsartan-containing products, the recall also includes certain losartan and irbesartan products as well, according to an FDA questions and answers page.
Lawyers are filing valsartan lawsuits on behalf of people who were diagnosed with cancer after taking valsartan for more than a year or who suffered liver or kidney injuries that required an emergency room visit or hospital stay.
NDMA Cancer Risk
The FDA recalled some valsartan products because it found traces of NDMA, a chemical that can cause tumors in animals and may potentially cause cancer in humans. Cancer is not a typical valsartan side effect.
“We estimated that if 8,000 people took the highest valsartan dose (320 mg) from NDMA-affected medicines daily for four years (the amount of time we believed the affected products had been on the U.S. market), there may be one additional case of cancer over the lifetimes of these 8,000 people beyond the average cancer rate among Americans,” FDA Commissioner Scott Gottlieb said in a statement.
On Feb. 28, 2019, the FDA posted its interim limits for NDMA, NDEA and NMBA in angiotensin II receptor blockers. The FDA calculated its acceptable limits based on a 1 in 100,000 cancer risk after 70 years of exposure. The FDA will use these limits to inform recalls of contaminated products.
- NDMA — 96 nanograms per day; 0.3 parts per million
- NDEA — 26.5 nanograms per day; 0.083 parts per million
- NMBA — 96 nanograms per day; 0.3 parts per million
Timeline of the Valsartan Recall
The FDA hasn’t given any updates on the valsartan recall since 2019 when it issued a warning to Mylan Pharmaceuticals for not adhering to current good manufacturing practice (CGMP) in November.
From 2018 to February 2023, manufacturers recalled more than 18 million bottles of valsartan and other contaminated blood pressure drugs, according to USA Today.
- February 28, 2019: FDA posted acceptable intake limits for NDMA, NDEA or NMBA in valsartan, losartan, ibesartan, azilsartan, Olmesartan, eprosartan, candesartan and telmisartan.
- October 5, 2018: The FDA posts lab results of NDMA levels in valsartan products.
- September 28, 2018: The FDA placed ZHP on an import alert banning the drug maker from importing any more drugs into the US.
- September 13, 2018: The FDA and the European Medicines Agency discover that Zhejiang Huahai Pharmaceuticals (ZHP) found another NDMA-like contaminant, NDEA, in several batches of its valsartan active ingredient.
- August 9, 2018: FDA found Hetero Labs Limited of India, marketing under the Camber Pharmaceuticals label, also manufactured contaminated valsartan. Hetero used a similar manufacturing process to ZHP.
- July 13, 2018: The FDA issued a voluntary recall for drugs containing contaminated valsartan. Major Pharmaceuticals, Solco Healthcare and Tevan Pharmaceuticals recalled products with active ingredients supplied by Zhejiang Huahai Pharmaceuticals (ZHP), Linhai, China.
- July 5, 2018: European countries begin recalling contaminated valsartan.
Since the valsartan recall, manufacturers have recalled other drugs with NDMA impurities. In 2020, FDA requested the removal of all Zantac made with ranitidine from the market for NDMA contamination.
In the case of Zantac, FDA found levels of NDMA increased in the drug over time and when stored at higher-than-normal room temperatures. People began filing Zantac cancer lawsuits shortly after the recall.
Which Valsartan Drugs and Manufacturers Are Part of the Recall?
The valsartan recall includes several, but not all, generic drugs that contain valsartan as the active ingredient. The recall does not currently include the brand name valsartan-containing drugs Diovan, Entresto, Exforge, and Exforge HCT.
- Amlodipine and valsartan
- Amlodipine, valsartan and hydrochlorothiazide (HCTZ)
- Valsartan and hydrochlorothiazide (HCTZ)
Only specific valsartan manufacturers recalled the drug. The FDA said it will update its list as it continues to investigate.
- A-S Medication Solutions LLC (Teva/Actavis & Prinston/Solco)
- American Health Packaging (Aurobindo)
- Aurobindo Pharma USA, Inc.
- Aurobindo Pharma USA, Inc. (Acetris)
- AvKARE Inc. (Hetero/Camber)
- AvKARE Inc. (Teva/Actavis)
- Bryant Ranch Prepack Inc. (Teva/Actavis)
- Hetero Labs Inc. labeled as Camber Pharmaceuticals Inc.
- H J Harkins Company Inc. (Prinston/Solco)
- Mylan Pharmaceuticals Inc.
- Northwind Pharmaceuticals (Teva/Actavis)
- NuCare Pharmaceuticals Inc. (Prinston/Solco)
- Preferred Pharmaceuticals Inc., labeled as Solco Healthcare LLC
- RemedyRepack Inc. (Prinston/Solco)
- RemedyRepack, Inc. (Torrent)
- RemedyRepack Inc. (Hetero/Camber)
- Rising Pharmaceuticals Inc. labeled as Acetris Health LLC (Aurobindo)
- Solco Healthcare LLC (Prinston)
- Teva Pharmaceuticals USA Inc.
- Teva Pharmaceuticals USA Inc. labeled as Actavis Pharma, Inc.
- Teva Pharmaceuticals USA Inc. labeled as Major Pharmaceuticals
- Torrent Pharmaceuticals Limited
For updates on drugs included in the valsartan recall, please check the FDA’s list of recalled drugs on its website.
How to Find Out If Your Drug Is Part of the Valsartan Recall
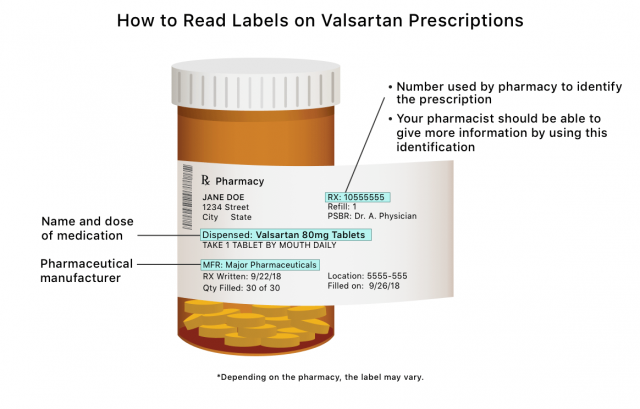
Anyone taking valsartan or a combination drug affected by the valsartan recall should locate the name of the drug’s manufacturer and the lot number either on the prescription bottle label or in the warning insert that accompanies the medication. Then, check with your pharmacist to see if your medication is one the valsartan recall list or search the FDA’s recall list for the manufacturer and lot number.
The FDA also uses a National Drug Code to track products. The FDA’s list includes the NDC codes for all recalled valsartan products. Normally, your prescription bottle from the pharmacy will not contain this information. Your pharmacist should be able to check these codes for you.
What Should I Do If My Drug Is Recalled?
If your valsartan manufacturer is a part of the recall, do not stop taking your medication. Suddenly stopping your medication may lead to more immediate health problems. First, talk to your doctor about your treatment options.
Next, search for your drug’s manufacturer in the FDA’s Drug Recalls database. Some manufacturers provide guidance in the event of a medication recall. For example, Torrent Pharmaceuticals Limited lists all the lots and products included in its recall. It also provides contact information and instructions for patients.
If you cannot find information on the FDA’s website, talk to your pharmacist. Don’t throw away your medication until you have your new medication. Your pharmacist can offer instructions for disposing of old medication.
Alternatives to Valsartan
Several blood pressure drugs may be a suitable alternative to valsartan. There are also drugs in the same class as valsartan that are not currently part of the recall. You may also be able to get valsartan from a manufacturer that is not on the recall list.
Ask your doctor about your treatment options. Each of these medications has its own risks or side effects.
- Azilsartan
- Candasartan
- Eprosartan
- Irbesartan
- Losartan
- Olmesartan
- Telmisartan
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.







