Vioxx Lawsuits
Vioxx lawsuits claimed the drug increased the risk of cardiovascular problems, such as heart attack, stroke and death. Merck voluntarily removed the drug from the market and settled about 60,000 Vioxx claims for $4.85 billion. Merck also faced and settled criminal charges and investor lawsuits.
Article Continues Below
Drugwatch.com content is legally reviewed for accuracy and quality.
Examples of trusted legal reviewers include qualified mass torts lawyers and certified paralegals.
Drugwatch.com writers gather lawsuit information by studying court records, watching lawsuit proceedings and speaking with experienced attorneys.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- MDL
- Personal Injury: Eastern District of Louisiana, MDL No. 1657 (not active); Securities: District of New Jersey, MDL No. 1658 (closed)
- Settlements
- $4.85 billion and several smaller settlements
Vioxx Lawsuit Status
As of April 2025, there have been no new developments in this litigation since Merck settled these cases in 2007 for $4.85 billion. Our legal partners and most lawyers are no longer taking Vioxx cases.
Merck voluntarily took Vioxx (rofecoxib) off the market in 2004, just five years after the popular prescription arthritis drug was introduced. Studies linked it to thousands of cases of heart disease and stroke.
Vioxx lawsuits were consolidated into multidistrict litigation in 2005. The first lawsuit ended in a $253 million verdict for the widow of a man who died after taking Vioxx. However, the award was later overturned. Merck eventually settled all cases for $4.85 billion.
Merck settled Department of Justice criminal charges for $950 million in 2011. The company settled a class action lawsuit filed by investors for $830 million in 2016.
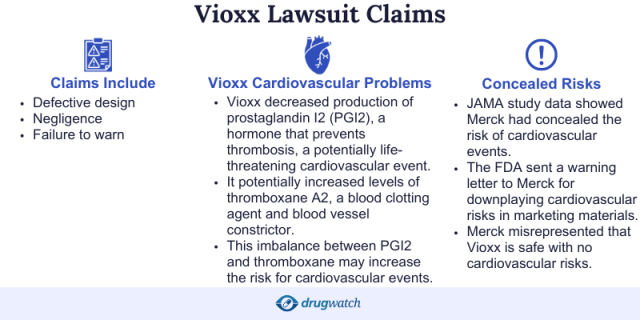
Why Did People File Vioxx Lawsuits?
People filed Vioxx lawsuits because they claimed the drug caused them to suffer from heart attacks and strokes, which were sometimes fatal. According to the lawsuits, Merck knew about the risks but failed to warn the public.
Plaintiffs claimed Vioxx was defective and dangerous and that it lacked proper warnings, which made it unfit to be marketed and sold. Damages claimed in lawsuits included wrongful death damages, survival damages, reimbursement for the cost of purchasing Vioxx, punitive damages, legal fees and coverage for past and future medical expenses.
Vioxx Lawsuit Case Study
Carol Ernst v. Merck
Carol Ernst became the first of tens of thousands of consumers who sued Merck over allegations that the company had concealed information about the serious health risks of the popular arthritis drug — including the risk of fatal heart attacks and strokes — in an effort to protect sales.
Vioxx Usage:
Robert Ernst, a marathon runner and fitness fanatic, started taking Vioxx (rofecoxib) in November 2000 to treat pain in his hands.
Injuries Alleged:
Less than eight months later, the 59-year-old Texas man died of a heart arrhythmia that he experienced in his sleep. Ernst’s widow, Carol, couldn’t understand what caused her husband’s sudden death. The more she researched it, the more she suspected the culprit was Vioxx, a nonsteroidal anti-inflammatory drug (NSAID) that had been heavily advertised for its easy-on-the-stomach pain relief.
Relief Sought:
General damages, wrongful death damages, loss of consortium, punitive damages
Verdict:
The jury awarded $253 million to Carol Ernst. However, under Texas tort laws that impose caps on damages, that award was reduced and later overturned altogether by a court of appeals that found there was insufficient evidence to determine that Robert Ernst’s heart attack was directly caused by his use of Vioxx.
Cardiovascular Risks Suspected for Years
Merck executives became aware of potential cardiovascular risks associated with Vioxx as early as May 2000, but they did little to pull back on the drug’s development and marketing. Clues about troubling side effects emerged in a large clinical trial launched in 1999 that compared the gastrointestinal side effects of Vioxx with side effects of naproxen, an older painkiller.
As Merck had hoped, results from the Vioxx Gastrointestinal Outcomes Research study (VIGOR) showed that patients taking Vioxx suffered fewer ulcers and bouts of gastrointestinal bleeding. But during the study, 79 of the 4,000 patients taking Vioxx suffered serious heart problems or died — a number nearly twice as high as in the naproxen group.
“Research later published in the medical journal Lancet estimates that 88,000 Americans had heart attacks from taking Vioxx, and 38,000 of them died.”
Despite the red flags, marketers at Merck decided against a study aimed specifically at Vioxx’s effects on the heart. They feared it might send the wrong message and interfere with Merck’s competitive edge on the rival drug, Celebrex.
“Research later published in the medical journal Lancet estimates that 88,000 Americans had heart attacks from taking Vioxx, and 38,000 of them died,” according to an NPR report published after Vioxx was recalled in 2004.
Studies Reveal Risks, Recall Follows
More troubling information emerged in 2001, when Dr. Eric J. Topol and other cardiologists at Cleveland Clinic published a report in the Journal of the American Medical Association (JAMA) alleging that COX-2 inhibitors such as Vioxx appeared to increase the risk of cardiovascular events.
Merck said the report was flawed and dismissed the call for a clinical trial aimed specifically at investigating cardiovascular risks. Topol reported that, prior to the report’s publication, scientists from the billion-dollar drug company requested that the information be kept from the public.
“Sadly, it is clear to me that Merck's commercial interest in rofecoxib sales exceeded its concern about the drug's potential cardiovascular toxicity.”
Merck officials denied that claim but in 2002 changed their label to warn about higher risks of heart attack. The company finally decided to pull Vioxx from shelves in 2004, after additional studies revealed an increased risk of strokes and heart attacks among patients taking the drug for 18 months or longer.
After Vioxx was eventually pulled from the shelves, Topol wrote an article published in the New England Journal of Medicine stating that “there may be tens of thousands of patients who have had major adverse events attributable to rofecoxib.”
“Sadly, it is clear to me that Merck’s commercial interest in rofecoxib sales exceeded its concern about the drug’s potential cardiovascular toxicity,” he wrote.
Vioxx Settlements
Despite wins in multiple individual trials, Merck eventually settled the heart attack injury cases in 2007 for $4.85 billion without admitting fault. Following that settlement, the company settled criminal charges with the DOJ as well as claims of illegal marketing and shareholder lawsuits.
DOJ Investigations, Plea Deals and Settlements
Merck agreed to pay another $950 million to the DOJ in 2011 to resolve criminal charges and civil claims related to its alleged illegal promotion and marketing of Vioxx.
Under the terms of the settlement, Merck also agreed to plead guilty to a misdemeanor charge of a single violation of the Federal Food, Drug and Cosmetic Act for introducing a misbranded drug into interstate commerce, and they were ordered to pay a criminal fine of $321 million.
A secondary civil settlement required an additional payout from Merck of $628 million to resolve allegations regarding off-label marketing of Vioxx and false statements about the drug’s cardiovascular safety. The payout was to be divided between the U.S. and participating Medicaid states based on claims that Merck’s illegal marketing practices had influenced doctors to prescribe a drug they would not have otherwise prescribed.
The misbranding charge resulted from Merck’s promotion of Vioxx as a treatment for rheumatoid arthritis before the FDA had even approved it for that use.
Shareholder Lawsuits End in $830 Million Settlement
Over a decade after Merck withdrew Vioxx from the market, they agreed to pay yet another $830 million to settle a class action suit spurred by disgruntled investors.
Shareholders claimed that Merck’s misleading statements regarding the arthritis painkiller, including the results of the VIGOR study, and the way the company downplayed the drug’s risks had influenced them to make a bad investment. This, they claimed, caused them to incur significant losses when the drug was pulled from the market.
Despite its decision to remove Vioxx from the health care market, Merck continues to deny any liability or wrongdoing for claims brought against the company.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


