DePuy ASR Hip Recall
DePuy Orthopaedics recalled its ASR Hip Resurfacing System and ASR XL Acetabular System in 2010 after studies showed that the hip implants had high failure rates. The devices were recalled from the Australia market in 2009, and several experts have criticized how DePuy delayed the international recall.
ASR XL Acetabular System and ASR Hip Resurfacing System
DePuy Orthopaedics recalled its Articular Surface Replacement systems from the worldwide market in August 2010. In its recall statement, DePuy cited registry data that indicated significant issues.
Data from the National Joint Registry of England and Wales indicated 13% of XL Acetabular implant patients needed revision surgery within five years, a strikingly high rate compared to the expected lifespan. About 12% of Hip Resurfacing implant patients required revision surgery within five years. The expected lifespan for most hip implants is 15 years.
Initially, DePuy had claimed other sources of data indicated lower revision rates for ASR hip implants. Investigative journalists and claims in lawsuits, however, reported DePuy knew of high failure rates more than a year before the devices were recalled.
DePuy continued to sell other metal-on-metal hip implants after the ASR recall. The company no longer sells metal-on-metal components, however.
Who Did the Recall Impact?
ASR XL Acetabular System and ASR Hip Resurfacing System were implanted in more than 93,000 people worldwide. In the U.S., 30,000 people had the ASR XL Acetabular System implanted since 2003. The U.S. Food and Drug Administration declined, however, to approve the ASR Hip Resurfacing System in 2009, so the recall did not impact U.S. patients.
All-metal DePuy hip replacements, including the ASR models, have been linked to dangerous complications. When the metal components rub against one another, they release metal fragments that can cause metallosis, inflammation and systemic side effects.
DePuy stated at the time that it would “cover reasonable and customary costs of monitoring and treatment for services, including revision surgeries, associated with the recall of ASR,” in a 2010 statement. There were media reports at the time, however, indicating that the company denied some claims for medical costs. Johnson & Johnson, DePuy’s parent company, then settled thousands of ASR hip lawsuits for $4 billion in 2013.
Events Leading Up to Recall
Some experts believe that the ASR implants should have been recalled sooner than 2010. Patients reported complications that led to revision surgeries several years before DePuy pulled the metal hip implants from the market.
In Australia, health officials recalled the ASR products in 2009 based on data indicating a significantly high failure rate. In March 2010, DePuy sent a letter to doctors advising them of the high failure rates and particular risks to women and people with compromised bone quality.
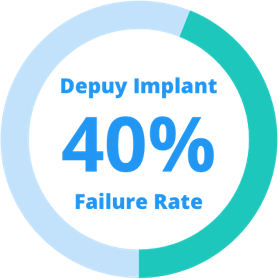
According to legal documents, DePuy was aware of the failure risks of its hip implants. Failure rates in some of the company’s studies were close to 40%. The company continued, however, to sell the devices before issuing the recall.
Managing the Recall Process
DePuy hired Broadspire Services to handle patients’ out-of-pocket medical expense claims. Broadspear doctors reportedly denied revision surgery claims despite recommendations from patients’ doctors.
“Doctors who are evaluating these cases are being paid indirectly by DePuy, and research suggests that even when we are very well-intentioned we can be influenced by conflicts of interest,” Kristin Smith-Crowe, who specializes in business ethics at the University of Utah, told Reuters.
“This was a bit of a red flag in terms of the way this situation was set up.”
DePuy also reached out to doctors about sending informational packets regarding the recall and the claims process directly to patients. The company urged patients to contact DePuy directly to make the claims process more efficient and established a website about the recall.
Critics allege the company was trying to collect patient data for use in potential lawsuits. Lawyers also say DePuy was trying to settle legal claims before potential claimants contacted an attorney.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




