Allergan Breast Implant Lawsuits
Allergan breast implant lawsuits claim Allergan Inc. and its subsidiaries did not properly warn women that their textured breast implants carried a risk of a type of cancer called breast implant-associated anaplastic large cell lymphoma, or BIA-ALCL.
Article Continues Below
Drugwatch.com content is legally reviewed for accuracy and quality.
Examples of trusted legal reviewers include qualified mass torts lawyers and certified paralegals.
Drugwatch.com writers gather lawsuit information by studying court records, watching lawsuit proceedings and speaking with experienced attorneys.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
Latest Allergan Lawsuit Updates
As of April 2025, there were 1,261 pending lawsuits in multidistrict litigation (MDL) 2921 before U.S. District Judge Brian R. Martinotti in New Jersey.
-
December 2024:
There is not much movement in the MDL to report on over the last few months. Case management conferences have been regularly cancelled since July. We will continue to monitor the progress of the litigation and provide updates here.
-
August 2024:
From our review of the MDL docket, 2:19-md-2921, Judge Martinotti cancelled the Case Management conference scheduled for Aug. 13, 2024. He did not suggest a new date. The parties have until the end of the month to select bellwether test trial cases for discovery. Hopefully, this means we will soon have trial dates to report on.
-
May 2024:
Judge Martinotti set MDL case management conference dates from June through December 2024. The New Jersey Multicounty Litigation has a discovery conference set for July 9, 2024.
-
March 2024:
No new cases have been added to the MDL since December 2023. The next status conference was rescheduled from March 7, 2024 to May 6, 2024.
-
December 2023:
There have been no bellwether trials or global settlement in the Allergan Breast Implant MDL. Lawyers are continuing to accept and file lawsuits on behalf of people injured by the devices.
-
October 2023:
Judge Martinotti signed an order on Oct. 5 allowing the Plaintiffs’ Executive Committee to change the composition of the Plaintiffs’ Steering Committee.
-
May 2023:
Special Master Joseph A. Dickson set a deadline for depositions of putative class representatives for September 18, 2023.
-
March 2021:
Judge Martinotti granted Allergan’s motion to dismiss only in part and has allowed the cases to remain active in the MDL.
-
February 2020:
Allergan filed a motion to dismiss all plaintiffs’ complaints.
-
December 2019:
Judges transferred Allergan lawsuits into an MDL in New Jersey.
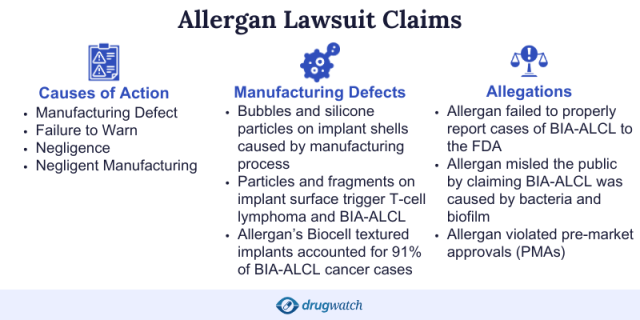
On July 24, 2019, the Food and Drug Administration announced that it had found evidence that Allergan textured breast implants had a higher risk of Breast Implant Associated Lymphoma than other textured implants.
The manufacturer issued a worldwide recall for several brands of its Biocell textured breast implants after the FDA requested the company pull the devices from the United States market. Allergan’s subsidiaries Inamed and McGhan manufactured some of the recalled devices.
Allergan Breast Implant Cancer Claims
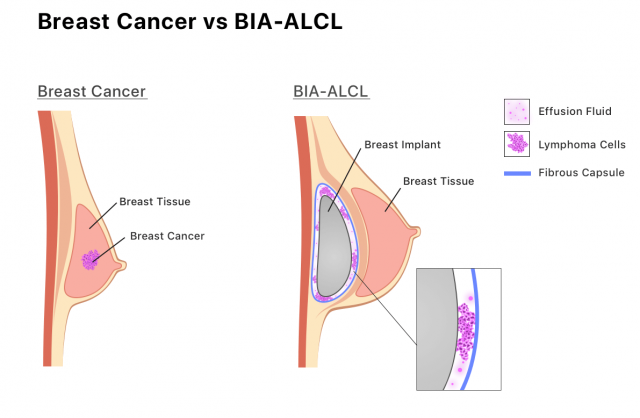
The main injury claimed in Allergan breast implant lawsuits is a rare type of cancer called breast implant-associated anaplastic large cell lymphoma, or BIA-ALCL. So far, the majority of reported cases worldwide involved Allergan’s textured implants, according to the FDA.
BIA-ALCL is not breast cancer. It is a type of non-Hodgkin lymphoma. These cancerous cells develop in scar tissue and fluid near the implant, and in some cases it can spread and lead to death. However, in September 2022, the FDA issued a safety communication warning the public that types of cancer other than BIA-ALCL have been found in the scar tissue that surrounds smooth and textured implants.
Treatment requires surgery to remove the implant and affected tissue. In some cases, patients require radiation and chemotherapy. Although the disease is treatable, it can still cause trauma in patients.
“We cannot allow women to be unnecessarily harmed while we collect data,” Dr. Eric Swanson wrote in a February 2019 article in the Annals of Plastic Surgery. “Capsulectomies, positron emission tomography and computed tomography, the cost of medications (brentuximab is very expensive), and the emotional and financial impact of BIA-ALCL take a heavy toll on affected women, even if the disease is seldom fatal.”
Swanson wrote that some plastic surgeons feel it is time to abandon textured implants. He cited data from non-industry funded studies showing Allergan’s Biocell textured implants have more complications.
“The emotional and financial impact of BIA-ALCL take a heavy toll on affected women, even if the disease is seldom fatal.”
In addition to the BIA-ALCL risk, these implants also had greater incidences of seromas, or fluid pockets, compared to other implants. They also carried a greater risk of performance failure, pain, rupture and scar tissue around the implant known as a capsule.
Symptoms of BIA-ALCL
Symptoms of BIA-ALCL mainly include persistent swelling or pain around the breast implant. These symptoms can occur several years after receiving the implant.
A doctor may find evidence of fluid collection around the implant or capsular contracture, which occurs when scar tissue squeezes the implant. Some women have reported lumps in their breasts.
When Did the FDA First Know About the Risk?
The FDA first identified a possible association between breast implants and anaplastic large cell lymphoma in 2011. But the agency said there were too few cases at the time to properly gauge the risk.
In 2016, the World Health Organization classified the disease as a type of T-cell lymphoma that developed after receiving breast implants. In 2017, the FDA announced BIA-ALCL was primarily associated with textured implants.
The FDA held a public advisory committee meeting in March 2019 to discuss risks. Initially, the agency declined to recommend a recall. Then new data in July 2019 showed a spike in BIA-ALCL cases.
This data led to the July 2019 Allergan textured implant recall.
How Many Women Have Been Affected?
In August 2022, FDA reported that it had received 1,130 reports of BIA-ALCL as of April 2022. Of the 1,130 cases of BIA-ALCL reported worldwide, 953 — or 84 % — were reported for Allergan implants.
As of the most recent update in June 2023, reports climbed to 1,264. Of those reports, 1,079 involved an Allergan implant — or 85%.
The FDA received 63 reports in which a patient had died, as of June 2023. Thirty-seven of those patients had an Allergan breast implant at the time of their BIA-ALCL diagnosis.
- McGhan BioDimensional Silicone-Filled Biocell Textured Breast Implants
- McGhan Magna-Site Tissue Expander
- McGhan Style 134 Croissant Shaped Tissue Expander
- Natrelle 133 Plus Issue Expanders
- Natrelle 133 Tissue Expanders with and without suture tabs
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants
- Natrelle Inspira Silicone-Filled Breast Implants
- Natrelle Saline Breast Implants
- Natrelle Silicone-Filled Breast Implants
- Style 133 Biospan Tissue Expander
If you are unsure if you have a Biocell textured implant, check with your plastic surgeon.
Plaintiffs: Manufacturer Failed to Warn About Cancer Risk
Plaintiffs in Allergan’s textured breast implant lawsuits allege the devices caused women to develop BIA-ALCL. They further allege that the defendants failed to adequately warn against this risk and failed to promptly and properly report the results of the post-marketing studies relating to these products, according to Allergan’s 2018 annual report.
Two of the women who have filed lawsuits are Vivian Skelton and Sandra Rush.
Breast Implant Case Study #1
Vivian Skelton’s Allergan Natrelle Silicone Breast Implant Lawsuit
Vivian Skelton filed her lawsuit against Allergan and its subsidiaries in March 2018 in California.
Breast Implant Use:
She had Allergan Natrelle Silicone Breast Implants implanted in May 2014.
Injuries Claimed:
After the surgery, she experienced pain, discomfort, fatigue and swelling in her left breast. Throughout the two years that followed, she suffered constant pain and fluid buildup. She went through several MRIs and ultrasounds and two revision surgeries.
“[Allergan] disseminated false information, in that they engaged in false and misleading sales and marketing tactics, touting the aesthetic beauty of breast augmentation and minimizing the risks,” Skelton’s lawsuit claimed.
Ultimately, an MRI in February 2016 revealed skin thickening, swelling and cysts up against the implant. Doctors diagnosed her with BIA-ALCL and removed the implant. She underwent several rounds of chemotherapy and suffered several complications, including cognitive dysfunction that required her to stay in a rehab facility.
Accusations Against Allergan:
Skelton’s lawsuit claims Allergan “disseminated false information, in that they engaged in false and misleading sales and marketing tactics, touting the aesthetic beauty of breast augmentation and minimizing the risks, which reached physicians, the medical community, and the public with knowledge that the information was, in fact, false and misleading.”
Breast Implant Case Study #2
Sandra Rush’s Lawsuit Involving McGhan Textured Saline Implants
Sandra Rush filed her lawsuit against Allergan and its subsidiaries in May 2019 in New Jersey.
Breast Implant Use:
Rush had McGhan textured saline implants implanted in 1995.
Injuries Claimed:
In 2017, she noticed her left breast had become swollen and sore. After a mammogram and biopsy, doctors diagnosed her with BIA-ALCL. She had surgery to remove the implant and additional surgery to reconstruct her breast with her abdominal fat.
The cancer had spread, and she had to undergo treatment for stage 4 BIA-ALCL. Tests showed she had cancer cells in her spine, pelvis, jaw, bone marrow and skin.
She had five cycles of chemotherapy as well as a bone marrow transplant, and she had to leave her job. As a result, Rush suffered from depression, nausea and insomnia.
Accusations Against McGhan:
“Despite Defendants’ knowledge of an association between breast implants and ALCL dating back to the 1990’s, Defendants purposefully failed to comply with their clearly established post-market surveillance obligations and in doing so have exposed many hundreds of thousands of women to life-altering and avoidable cancer,” Rush said in her lawsuit.
Is There a Class Action?
Several Allergan breast implant class action cases have been filed and are among the actions that have been consolidated in a multidistrict litigation in New Jersey. These cases are not the same as the personal injury cancer cases.
In addition, the International Consortium of Investigative Journalists reported that investors have filed a class action lawsuit claiming Allergan plc headquartered in Dublin, Ireland, concealed the link between its textured implants and the rare type of lymphoma. The suit also claims Allergan failed to inform investors that the safety findings threatened regulatory approvals in Europe.
Shareholders Accuse Allergan of Fraud
Thomas F. Cook filed the class action in New York on Dec. 21, 2018. Cook’s fraud lawsuit claimed Allergan “employed devices, schemes, and artifices to defraud” shareholders and mislead them about its textured breast implants.
“Allergan misled investors by boasting about various ‘pharma and device approvals,’” Cook’s lawsuit claimed. “The truth was revealed on December 19, 2018, when the Company announced that it had suspended the sale of [textured breast implants and tissue expanders] and that it was withdrawing all remaining supplies from European markets.”
“Allergan misled investors by boasting about various ‘pharma and device approvals.’”
The lawsuit came after Allergan announced it had lost its textured implant CE Mark, a European safety certificate needed to sell its devices in 33 European countries.
The French accredited certification firm LNE G-MED did not renew the company’s CE Mark because it wanted to see further data from Allergan before re-issuing the safety certificate, the International Consortium of Investigative Journalists reported.
French Recalls
Meanwhile, French regulator Agence Nationale de Sécurité du Médicament asked Allergan to recall batches of affected products from all French hospitals.
“Allergan stands behind the benefit/risk profile of our breast implant products. The ANSM request, and this action, is not based on any new scientific evidence regarding these products,” Allergan said in a Dec. 19, 2018, press release regarding its decision to suspend sales and withdraw textured breast implants in Europe.
Questions Lawyers May Ask
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.





