Baby Formula: Controversies, Risks & Industry Failures
Infant formula plays a critical role in the health of millions of babies every year in the United States. But certain formula brands have been linked to the risk of a life-threatening condition called necrotizing enterocolitis (NEC) among premature infants, leading to lawsuits.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by Wilson & Peterson LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
I found Drugwatch to be very helpful with finding the right lawyers. We had the ...
- Legally reviewed by Brendan A. Smith, Esquire
- Last update: October 30, 2025
- Est. Read Time: 11 min read
- Why Is Baby Formula Under Scrutiny?
- The Science Behind Baby Formula & NEC Risks
- How Abbott & Mead Johnson Handled Formula Safety Concerns
- Government & Regulatory Responses to Baby Formula Risks
- The Ongoing Debate Over Safer Infant Formula Options
- What Can You Do if You Suspect Baby Formula Harmed Your Infant?
Why Is Baby Formula Under Scrutiny?
Infant formula plays a big role in providing essential nutrients for infants, especially when breastfeeding isn’t an option. Formulas are often fortified with beneficial ingredients to nourish infants, such as iron, vitamins and minerals.
According to the Food and Drug Administration, many infants rely on baby formula for some or all of their nutritional needs.
But concerns over safety, contamination and the risk of serious health issues have led to baby formula lawsuits.
Research has established a link between some cow’s milk-based formulas and necrotizing enterocolitis (NEC) in premature infants. NEC is a serious and potentially fatal intestinal condition where bacteria can inflame and infect intestinal tissue, leading to perforation and infection.
There are currently hundreds of pending lawsuits alleging that baby formula manufacturers Abbott Laboratories and Mead Johnson failed to warn parents of potential NEC risks associated with some of their cow’s milk formulas.
The Link Between Baby Formula & Necrotizing Enterocolitis (NEC)
For years, research has suggested that a link may exist between formula feeding and NEC. According to the Cleveland Clinic, 90% of infants who develop NEC are born prematurely. There continues to be debate over what risk factors play the most significant roles in the development of the condition, but feeding remains widely regarded as a key indicator.
One of the first studies to link cow’s milk formula to NEC was a 1990 study published in the Journal of Medical Science, which showed that NEC was up to 10 times more common in formula-fed premature babies.
A 2018 critical analysis of NEC risk factors consulted 35 NEC experts and asked them to review 64 different potential risk factors. From that list, formula feeding vs. breastfeeding was one of just three NEC development risk factors that experts highly agreed on. The condition typically occurs within the first two weeks of life in premature infants who are fed specific formulas.
Not every type of baby formula is tied to NEC. Concerns generally involve certain cow’s milk-based formulas.
How Baby Formula Recalls Exposed Safety Issues
In addition to the potential threat of NEC, contamination issues and baby formula recalls have fueled health concerns surrounding infant formula.
Rarely, a type of bacteria called Cronobacter sakazakii, a germ that can survive in dried foods, may contaminate baby formula. This can happen during manufacturing, home preparation or during feeding from unsanitary bottles.
When Cronobacter infects infants, it can lead to the development of meningitis. At the time of the baby formula recalls, four hospitalizations and two deaths had been reported.
In February 2022, Abbott issued a baby formula recall for certain lots of its Similac, Alimentum and EleCare formulas after three complaints of Cronobacter sakazakii infection and one complaint of an infection by Salmonella in infants who consumed the formula.
Later that month, Abbott expanded the recall after the reported death of at least one infant infected with Cronobacter sakazakii. In March 2022, FDA inspectors observed several zones that tested positive for the presence of Cronobacter sakazakii in the Abbott factory that manufactured the affected formulas.
What Are the Long-Term Health Risks of Contaminated or Unsafe Formula?
The effects of unsafe baby formula can be wide-ranging and potentially devastating.
Contaminated formulas that contain germs like Cronobacter can be potentially fatal to infants. It can eventually cause meningitis, a condition in which the meninges, the linings of the brain and spine, begin to swell. Even non-fatal infections can cause serious and long-term issues in the brain.
NEC can also be fatal or cause long-term, potentially irreversible damage. Some infants require surgery due to the effects of NEC, with it becoming necessary to remove dead tissue from the abdomen.
The Science Behind Baby Formula & NEC Risks
Multiple studies have shown that cow’s milk-based formulas may result in a heightened risk of developing the serious condition.
According to a 2022 study published in Frontiers in Pediatrics, “Formula feeding is an important risk factor for the development of necrotizing enterocolitis in preterm infants.” However, the study cautions, “The potential harmful effects of different preterm formulas on the developing intestinal tract remain incompletely understood.”
What Studies Have Found About Formula & NEC
In the Frontiers in Pediatrics study, researchers noted “alterations in gut microbial composition” along with gut injury, inflammation issues and apoptosis when mouse pups were fed different types of formula.
A 2017 review published by the American Society for Nutrition determined that feeding cow’s milk formulas to premature infants “significantly increases the risk of NEC.”
- Frontiers in Pediatrics, 2022: Mouse pups experience gut injury and inflammation when fed specific types of formula.
- Critical Analysis of NEC Risk Factors, 2018: Many experts agree that formula is a key factor in the development of NEC when compared to breastfeeding
- American Society for Nutrition, 2017: Cow’s milk formulas significantly increase the risk of NEC.
Premature infants fed only breast milk experience lower rates of NEC. What remains unclear is exactly why this potential connection exists.
Why Did Manufacturers Continue Selling Formula Despite NEC Risks?
The makers of Similac and Enfamil have continued to push back on claims that their formulas are linked to the development of NEC, even as some juries have awarded multimillion-dollar verdicts to the families of infants who developed the condition.
“There is no scientific evidence showing Abbott’s preterm infant products cause or contribute to causing NEC,” Abbott spokesperson Scott Stoffel claimed following a 2024 trial loss, per KMOV. “Specialized formulas and fortifiers, like the one in this case, are part of the standard of care by the medical community and, along with mother’s milk and donor human milk, are the only available options to feed premature infants.”
How Abbott & Mead Johnson Handled Formula Safety Concerns
Though Abbott and Mead Johnson have recalled formula products, no recall was issued due to specific NEC concerns. The companies publicly maintain that their products are not associated with NEC risks.
After an NEC trial verdict in Mead Johnson’s favor, the company released a statement saying, “Where human milk is unavailable or when supplementation is necessary, specialized preterm hospital nutrition products can provide essential, lifesaving nutrition.”
What Did Baby Formula Manufacturers Know About NEC Risks?
According to Bloomberg, a plaintiff lawyer in one baby formula trial claimed that internal documents showed that Abbott officials had privately acknowledged that formula feeding was connected to NEC as early as 2009.
Both Similac and Enfamil publicly reject claims that their formulas are tied to NEC.
Government & Regulatory Responses to Baby Formula Risks
Though the FDA does not directly approve infant formulas, it does issue regulatory requirements for various activities, including the labeling and packaging of formula products. However, it has not had a significant response to potential links between NEC and certain kinds of formulas.
Still, other government entities have warned of the potential risks. In 2011, the U.S. Surgeon General released a call to action highlighting NEC’s possible ties to baby formula in a call to action to support breastfeeding.
The report noted that formula feeding for premature infants is associated with a higher risk of NEC.
Have Federal Investigations Been Launched Into Baby Formula Safety?
The federal government has launched several investigations into baby formula safety, largely in response to the recall, shortages and factory shutdown in 2022.
The Justice Department opened a criminal investigation in January 2023 into the Abbott facility that was shut down for several months following numerous issues.
Congressional hearings were also held over the nationwide infant formula shortage, which was due in part to the Similac recalls and shutdown of the Michigan plant.
In the wake of those developments, experts have also called for stricter regulation of infant formula. In February 2023, experts from institutions across the globe published a three-paper series highlighting concerns about the exploitative marketing of infant formula products, among other related issues.
In March 2025, the U.S. Department of Health and Human Services and the FDA announced Operation Stork Speed. The initiative is intended to ensure the safety, quality, availability and nutritional adequacy of baby formulas.
FDA actions include reviewing nutrients, increased testing for heavy metals and contaminants, encouraging more communication with manufacturers, encouraging more research on the outcomes of formula feeding and communicating with consumers to ensure transparency.
The Ongoing Debate Over Safer Infant Formula Options
Despite the risks and concerns tied to some types of infant formulas, formula as a whole remains a critical product for infant nutrition. Millions of infants in the U.S. are fed formula every year, and breastfeeding isn’t always a feasible option for families. Safe infant formulas can help meet infant nutritional needs.
What Are the Safest Baby Formula Options?
To find the safest baby formula option for your family, look for brands that are highly reputable, check for recalls and read about potential side effects or issues from different sources. Most importantly, be sure to talk to your doctor if you have concerns or questions about your baby’s nutrition.
Has the Baby Formula Industry Changed Its Safety Practices?
Safety has improved in the infant formula industry, particularly following the recalls and shortages of 2022.
Following the closure of the Michigan facility, the FDA implemented numerous changes to its inspection process and standards for infant formula facilities.
A new process for notifying Congress about infant formula recalls was implemented. Particular emphasis was placed on preventing Cronobacter contamination and infection, with the FDA altering or implementing a number of policies to improve testing and detection.
What Can You Do if You Suspect Baby Formula Harmed Your Infant?
If you suspect that your infant may have been harmed after being fed formula, consider reaching out to a lawyer who has experience with baby formula litigation. Hundreds of lawsuits have been filed by families and parents of infants affected by NEC.
How to Tell If Your Baby Was Harmed by NEC or Contaminated Formula
NEC primarily affects premature infants, and its symptoms can vary depending on the severity of the case. Signs of NEC can include diarrhea, blood in stool, poor feeding tolerance, lethargy and a swollen, bloated or discolored stomach. Changes in heart rate, breathing or blood pressure could also occur.
If your infant is in the NICU, the staff will likely inform you if your child has developed NEC. If you notice signs of NEC while your child is at home, seek medical care immediately.
The condition is generally diagnosed through X-rays, blood tests or fecal tests.
Contaminated baby formula can lead to a Cronobacter infection. Early signs of Cronobacter infection in infants can include a fever, low energy and excessive crying. Jaundice, grunting breaths, temperature changes and abnormal body movements may also be symptoms.
The initial identification of health complications stemming from contaminated formula may fall on the parents since these conditions can emerge when the infant is already living at home.
What to Do If You’re Concerned About Your Baby’s Formula
If you’re concerned about your baby’s formula, start by checking its expiration date and consulting the FDA’s website to determine if it’s part of a recall.
Always inspect the container for damage, unusual smells or changes in texture. If your baby shows signs of discomfort, such as vomiting, diarrhea or refusal to eat, consult your pediatrician.
If your infant is experiencing symptoms of NEC or Cronobacter, seek medical care as soon as possible.
Side Effects When Changing Baby Formulas
Sometimes, a pediatrician may recommend changing formulas due to food allergies, lactose sensitivity or other medical reasons. When switching baby formulas, some babies may experience mild side effects as their digestive systems adjust.
Common signs that a baby isn’t tolerating a new formula well include diarrhea, constipation, gas or frequent spitting up. More serious symptoms, such as bloody stools, congestion or wheezing after feeding, may indicate a formula intolerance or allergy and should be discussed with your pediatrician right away.
While not all babies experience side effects from switching formula, it’s important to monitor how your baby reacts and make changes gradually. Abruptly switching formulas can upset your baby’s sensitive digestive system. Instead, your pediatrician may suggest gradually mixing the new formula with the old one over several days to help your baby adjust comfortably.
If you notice any unusual symptoms or ongoing digestive issues, contact your pediatrician for guidance on how long to try the new formula and whether a different type may be needed.
Common and Serious Baby Formula Side Effects
Common baby formula side effects include eczema, upset stomach and runny nose. Side effects such as rash and digestive issues are most often caused by food allergies, contaminated formula or consuming improperly made formula.
Using an incorrectly made formula can also harm your baby. The CDC warns parents not to use homemade infant formula or buy imported formula online from third-party sellers because of potential problems like insufficient nutrition, dangerous electrolyte imbalances or contamination.
All baby formula legally sold in the United States must follow the guidelines for safety and nutrition of the FDA.
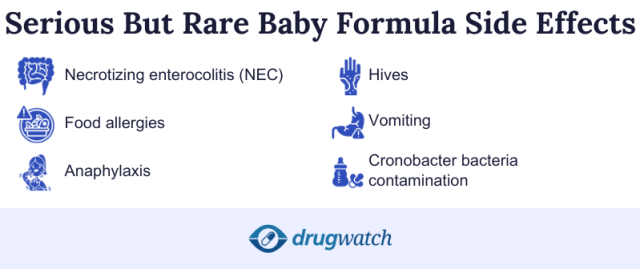
Baby formulas rarely cause serious side effects. However, if left unchecked, food allergies, nutrient deficiencies or baby formula contamination can lead to serious health problems.
Food allergy symptoms such as swelling of the mouth or throat, hives and vomiting are a medical emergency. These are uncommon and can happen within days to weeks of an infant starting cow’s milk baby formula. If your infant exhibits any of these symptoms, seek emergency medical attention immediately.
Baby formula has all the nutrition babies need. However, there are studies that show formula-fed babies may have a greater risk of obesity, respiratory infections, sudden infant death syndrome (SIDS), cognitive development issues, allergies and other health risks compared to breastfed babies.
Consult your pediatrician about a feeding plan that works best for you and your baby.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




