Eliquis: Side Effects, FDA Warnings & Lawsuit Information
Eliquis is a popular blood thinner used to prevent strokes and blood clots. While effective, it has ties to serious risks like excessive bleeding. FDA actions, label changes and product liability lawsuits highlight concerns over patient safety and a lack of adequate warnings.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: December 1, 2025
- Est. Read Time: 7 min read
What Is Eliquis?
Eliquis (apixaban) is a blood thinner medication (anticoagulant). It can reduce your risk of stroke and blood clots if you have a type of irregular heart rhythm called nonvalvular atrial fibrillation.
Doctors also prescribe the drug to treat blood clot disorders, including deep vein thrombosis and pulmonary embolism. Eliquis can even reduce your risk of blood clots following a hip or knee replacement.
Bristol-Myers Squibb and Pfizer manufacture Eliquis. No generic version will be available in the U.S. until 2028. However, generic versions are already on the market in other countries, including Canada.
Common Eliquis Side Effects
The most common side effect of Eliquis is bleeding. The risk of non-major bleeding is 2% to 4%, and the risk of major bleeding is 3% or less.
- Bleeding from cuts that takes longer to stop
- Bleeding from your gums or nose
- Bruising
- Dizziness
- Headache
- Heavy menstrual bleeding (in children)
- Nausea
- Vomiting (in children)
- Weakness
Some side effects may subside after your body adjusts to the medication.
If you have these complications with one blood thinner, that doesn’t mean you are guaranteed to have them with another. Discuss other options with your doctor if you experience bleeding issues with Eliquis.
Serious Side Effects of Eliquis
In clinical trials, major bleeding occurred in 2.13% of Eliquis users who had non-valvular atrial fibrillation. Rare and dangerous complications, such as bleeding within the skull, spine and the sac around patients’ hearts, were also reported.
- Blood clots or coughing up blood
- Brown, pink or red urine
- Epidural or spinal blood clots (hematoma)
- Fatal bleeding events
- Heavy vaginal bleeding
- Joint pain or swelling
- Permanent paralysis
- Red or black stools
- Severe allergic reactions
- Uncontrollable bleeding
- Vomiting blood
Severe allergic reactions to Eliquis can cause itching, hives, chest pain, swelling of your face or tongue, labored breathing and dizziness. These reactions are a medical emergency. If you experience them, call your doctor or 911 immediately.
Fortunately, Eliquis may be less likely to cause serious side effects than other blood thinners. For example, a study published in the Annals of Internal Medicine found that Eliquis has a lower rate of overall gastrointestinal bleeding than Xarelto (rivaroxaban).
Blood Clots with Early Discontinuation
According to the black box warning on Eliquis’s packaging, people who suddenly stop taking the drug are at a higher risk of developing blood clots.
The warning also specifies that nonvalvular atrial fibrillation patients in clinical trials were at a higher risk of stroke after stopping Eliquis. The label encourages these patients to switch to a different anticoagulant in order to minimize their risk of stroke.
Risk of Spinal or Epidural Blood Clots
People who receive spinal or epidural anesthesia or undergo spinal puncture while taking Eliquis are at risk of developing epidural or spinal blood clots. This can result in long-term injury or paralysis.
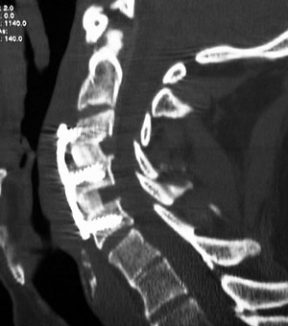

Taking Eliquis with other drugs that affect bleeding, like nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors or other anticoagulant drugs, can increase your risk of developing spinal/epidural blood clots. Repeated spinal or epidural punctures and use of indwelling epidural catheters also make these problems more likely.
If not treated swiftly and properly, a spinal or epidural blood clot can be deadly. Full recovery is possible, but hematomas of this nature can result in permanent damage to your brain.
Eliquis Side Effects in the Elderly
While no study has shown specific adverse reactions for seniors, elderly patients should discuss the risks of Eliquis with their doctor. Side effects for the elderly taking Eliquis include higher risks of bleeding complications, which could be aggravated by falls.
Elderly individuals with kidney impairment may need to adjust their dosage so the drug clears their system to avoid side effects. Patients with liver impairment should talk with their doctor about the correct dosage and possible side effects of Eliquis.
Eliquis Patient Safety Tips
To minimize your risk of side effects when taking Eliquis, watch for signs of bleeding and consult with your doctor before stopping the medication. Also, be careful when it comes to other medications or drinking alcohol while taking the drug.
- Don’t suddenly stop taking Eliquis without medical guidance. Doing so could increase your risk of stroke.
- Watch for signs of internal bleeding. If you have frequent nosebleeds, bleeding from your gums or blood in your urine or stool, call your doctor right away.
- Ask your doctor about reversal agents and emergency plans in the event of uncontrolled bleeding.
- If you have hip or knee replacement surgery, talk with your doctor to see if Eliquis is right for you to help prevent postoperative blood clots.
- Tell your doctor if you are pregnant or trying to become pregnant. Research is ongoing to determine if nursing mothers can pass Eliquis to babies through breast milk.
- Eliquis is not advised if you are prone to bleeding or have a history of liver or kidney disease.
- Drinking alcohol heavily while taking Eliquis may increase your risk of bleeding. If you plan on drinking, it’s wise to consume alcohol in moderation.
- Adverse drug interactions may occur with some antifungals, antibiotics, medications for abnormal heartbeat and anti-seizure medications. Be careful when taking NSAIDs or St. John’s Wort while taking Eliquis.
FDA Warnings and Regulatory Actions
The U.S. Food and Drug Administration (FDA) approved Eliquis in 2012 “to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.”
In 2014, the FDA also approved Eliquis to treat deep vein thrombosis and pulmonary embolism and to reduce the risk of blood clots following hip or knee replacement surgeries.
The FDA requires two black box warnings — the agency’s most serious alerts — for Eliquis. These advise patients of:
- A higher risk of blood clots if a patient suddenly stops taking the drug.
- An increased risk of hematomas resulting from spinal procedures.
These blood clots could lead to permanent paralysis.
FDA Approval Issues for Andexxa
In 2018, the FDA provided accelerated approval for a drug called Andexxa as a reversal agent of uncontrolled bleeding in Eliquis patients. However, in 2024, the FDA questioned Andexxa’s trial results, including its effectiveness and its increased risks for blood clots.
Andexxa remains available in the U.S. market.
Eliquis Clinical Trial Controversies
Eliquis blocks an enzyme called “Factor Xa,” preventing blood clots but increasing the risk of uncontrollable bleeding. When applying for FDA approval, Bristol-Myers Squibb offered data from its clinical trials, conducted between 2006 and 2011.
However, the documents revealed that some of the subjects received the wrong study drug. Additionally, some records had been improperly manipulated, and the hospital where the trial took place failed to report some adverse events. Bristol-Myers Squibb submitted additional data, and the FDA approved the drug in 2012.
Why Patients Are Filing Eliquis Lawsuits
Numerous patients and their families have filed lawsuits claiming that Bristol-Myers Squibb and Pfizer failed to adequately warn patients about the bleeding risks of Eliquis.
In addition, some lawsuits say that the manufacturers’ claim that Eliquis is safer and more effective than Warfarin (a competing blood thinner) lacks supporting evidence.
Eliquis is not the only blood thinner subject to lawsuits. Boehringer Ingelheim, the manufacturer of Pradaxa, has settled thousands of lawsuits claiming its drug caused serious complications, including severe internal bleeding.
Johnson & Johnson and Bayer, the manufacturers of Xarelto, have faced thousands of lawsuits claiming the company failed to warn consumers of the risk of potentially fatal internal bleeding.
Eliquis Lawsuit Updates and Settlement Information
In February 2017, the U.S. Judicial Panel on Multidistrict Litigation consolidated 53 pending Eliquis actions from 17 locations into one multidistrict litigation (MDL) in the Southern District of New York. The panel determined that the lawsuits shared the same factual and legal grounds in their complaints, forming the MDL to expedite the legal system.
However, in November 2017, District Court Judge Denise Cote dismissed these cases, saying that the Eliquis label adequately warns patients about its risks. The judge added that she found the plaintiffs’ arguments to be “unavailing.”
As of September 2025, there are no pending MDL cases involving Eliquis. To date, there have been no large public settlements of Eliquis cases.
However, there have been significant public settlements of other blood thinner lawsuits. In 2014, Boehringer Ingelheim settled roughly 4,000 lawsuits involving Pradaxa for $650 million. In 2020, the company settled another 2,935 cases. In 2019, Johnson & Johnson and Bayer paid $775 million to settle thousands of lawsuits involving Xarelto.
Alternatives and Related Drugs
While blood thinner drugs share many similarities, they also have some crucial differences. Here’s a look at how they compare to one another.
| Eliquis | Pradaxa | Warfarin | Xarelto | |
|---|---|---|---|---|
| Bleeding | X | X | X | X |
| Blood clots in spine | X | X | ||
| Bruising | X | X | X | |
| Dizziness | X | X | ||
| Nausea | X | X | X | X |
| Nosebleeds | X | X | ||
| Stroke | X | X | X |
Legal and Medical Support
If you are considering legal action regarding Eliquis, read our Eliquis lawsuits page for more information about your options. Drugwatch’s content is reviewed by board-certified medical experts, and our legal analysis comes from qualified mass tort attorneys.
As of September 2025, we are unaware of any lawyers currently accepting Eliquis lawsuits. However, you can contact an experienced product liability attorney to find out if your specific circumstances warrant an Eliquis lawsuit.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




