EpiPen Side Effects, Recalls & Lawsuit Information
EpiPens can save lives in allergic emergencies, but they’ve also been criticized over high costs and defective devices. Learn about EpiPen side effects, FDA warnings, recalls and the litigation alleging corporate misconduct.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Jessica Swirble, PharmD
- Last update: December 1, 2025
- Est. Read Time: 9 min read
What Is the EpiPen and Why Is It Controversial?
An EpiPen is an intramuscular auto-injector device that administers a medication called epinephrine. Epinephrine is used to treat anaphylaxis, a severe allergic reaction that can occur due to medications, food, insect stings and other allergens.
- Causes your heart to beat faster, increasing blood pressure and circulation
- Dilates your pupils
- Expands muscles in your lungs and airways to improve airflow and deepen breathing
- Increases blood flow in your muscles
- Prevents the release of histamine, which causes allergic symptoms
EpiPen prices have consistently increased over the years, causing public backlash and congressional hearings. Since then, EpiPen’s manufacturers and distributors have faced numerous lawsuits regarding high prices, product defects and unethical corporate practices.
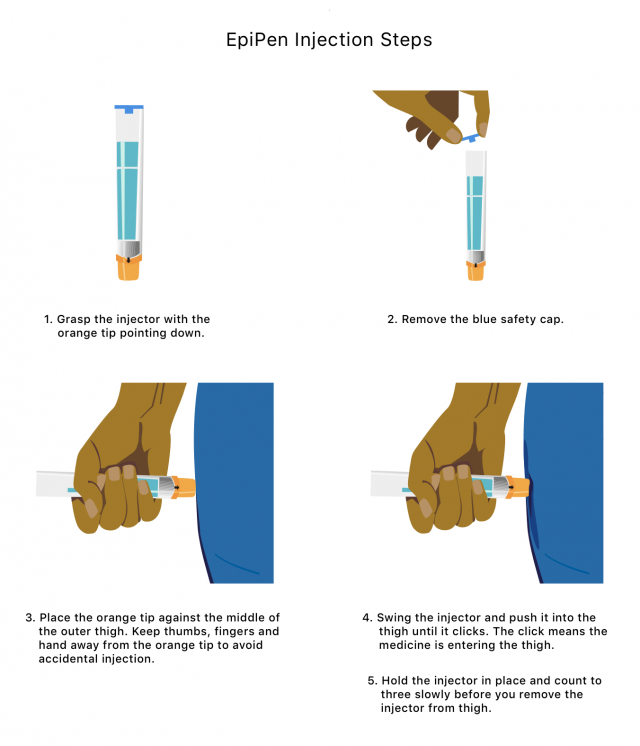
EpiPen Epinephrine Side Effects and Risks
Side effects from injecting epinephrine using an EpiPen are usually temporary. They can be mild, such as headaches and nausea, or serious, including heart rate changes or tissue infections.
After using an EpiPen, you should always seek medical care since further treatment may be necessary.
Common Side Effects
Common side effects from the epinephrine administered via an EpiPen include anxiety, dizziness, sweating, vomiting and more.
- Anxiety
- Breathing difficulties
- Dizziness
- Headache
- Increased heart rate
- Nausea
- Nervousness
- Pallor
- Palpitations
- Shakiness
- Sweating
- Vomiting
- Weakness
Few detailed clinical trials evaluate treating anaphylaxis with epinephrine. EpiPen’s label acknowledges this makes it “difficult to determine” just how often reactions to the drug occur. The label relies on less accurate observational data to determine common side effects.
Serious Complications
Although rare, the epinephrine in EpiPens can cause serious side effects, such as heart issues, blood glucose problems and kidney injuries. Severe complications are more likely to occur if you have preexisting conditions.
- Cardiac irregularities
- Heart attack
- Increased blood glucose levels
- Kidney problems
- Irritation, pain or redness at the injection site
Elderly patients or those with heart conditions, thyroid disease, high blood pressure, Parkinson’s disease or diabetes should have a conversation with their health care provider about their risks before using EpiPen.
Risks of Using Epinephrine After It Expires
Epinephrine in EpiPens has an estimated shelf life of 12 to 18 months. The biggest risk with expired devices is that they may be less effective or completely ineffective in an emergency.
A 2018 study found that epinephrine may still be effective years after the expiration date. Researchers from a separate 2019 study showed that epinephrine auto-injectors retain 100% of the drug at six months past the expiration date.
The 2019 study also found that every auto-injector in the study retained at least 90% of the drug up to 30 months after its expiration date. This suggests that patients may be able to use most auto-injectors safely even after they have expired.
Health care professionals still caution people to replace the devices before they expire to ensure effectiveness.
Device-Related Complications
Beyond the side effects of the epinephrine itself, device-specific problems, including accidental injections, malfunctions and failures, can lead to EpiPen complications.
- Accidental injection
- Allergic reactions to sulfite, a chemical added to the device to prevent the solution from browning
- Device failure
- Lacerations and bent or embedded needles in young children who resist during the injection
- Malfunctions
- Rare but serious skin and soft tissue infections
Accidental Injection
An accidental injection of epinephrine can cause numbness or tingling around your injection site. It may also increase your heart rate or, in rare cases, lead to tissue death.
- Increased heart rate
- Injection site injuries, including bruising, bleeding, discoloration or skeletal injury
- Local reactions at the injection site, including loss of feeling, coldness and pallor
- Loss of blood flow to hands or feet if injected into fingers or toes
- Tissue death
The EpiPen redesign in 2009 addressed the risk of accidental injections in part by modifying the design of the needle cover. With the newer devices, the needle is not exposed before or after use.
Device Failure or Malfunction
The U.S. Food & Drug Administration (FDA) has received reports of the EpiPen deploying needles too early or not at all. These types of malfunctions or failures can result in serious complications.
EpiPen’s manufacturer, Mylan, blamed a 2017 recall on a defective part that may cause the device to not activate properly. These failures were associated with seven deaths and 35 hospitalizations through mid-September that year.
In 2020, Mylan also revealed that sideways pressure on an EpiPen may cause the auto-injector to spontaneously activate when removing the safety release.
User Errors
EpiPens can be lifesaving, but it is important to use them correctly to prevent complications. You should only inject the EpiPen into the middle of your outer thigh to avoid complications.
Injecting an EpiPen into your finger or hand can cause your blood vessels to constrict, decreasing blood flow to the area. Injections into the buttocks have resulted in cases of a rare but fast-spreading bacterial infection that leads to tissue death called gas gangrene.
Additionally, children experiencing severe allergic reactions may not be able to remain calm or still to administer epinephrine. This has resulted in skin lacerations and injuries from bent or embedded needles. Caregivers of children must know how to immobilize a child’s leg to inject the EpiPen safely.
EpiPen Problems Reported to the FDA
The FDA has received thousands of reports of drug side effects and device complications for EpiPens. The four most noted complications to the FDA’s Adverse Events Reporting System (FAERS) were included in about 80% of all reports.
| Complications Reported | Cases Reported Between 1993 and 2017 |
|---|---|
| Accidental exposure | 734 |
| Device Failure | 877 |
| Drug Ineffective | 560 |
| Product Quality Issue | 575 |
As of November 5, 2025, the FDA had received 3,656 adverse event reports related to the devices since 1993. The agency categorized more than half as “serious cases.” These included 170 deaths, though the agency says the devices were not necessarily the cause of death.
FDA Warnings, Recalls and Government Actions
Reports of serious EpiPen complications after device failures between 2009 and early 2017 led to FDA warnings, government actions and recalls.
March 2017 Recall
In March 2017, Meridian Medical Technologies, Mylan’s EpiPen manufacturing partner, announced a voluntary recall of 13 lots of EpiPen products in the U.S. due to malfunctioning defects that resulted in the device failing to activate.
This recall initially affected over 80,000 defective devices shipped to Australia, Japan, New Zealand and various countries in Europe.
September 2017 FDA Warning Letter
In September 2017, the FDA sent a warning letter to Meridian outlining the process that led to Meridian eventually recalling 13 lots of EpiPens.
According to the FDA, Meridian had already inspected one of those 13 lots in June 2016, roughly nine months before its recall. Meridian closed the investigation without recommending a recall.
The FDA urged Meridian to re-examine the lot after inspecting Meridian’s manufacturing facility and observing “significant violations of current good manufacturing practice requirements.” It was only after Meridian performed this follow-up inspection that it detected the manufacturing flaws and recalled the faulty devices.
Additionally, the FDA noted that between 2014 and 2017, Meridian received 171 complaints regarding devices that failed to activate. However, Meridian did not inspect “the vast majority” of these failed devices.
The warning letter repeatedly states that Meridian inadequately responded to the violations the FDA observed during its inspection.
March 2020 FDA Alert
The FDA issued an alert in March 2020 to patients, health care professionals and caregivers regarding the potential for EpiPen device failure. EpiPen devices may fail to activate properly due to:
- Difficulty removing the device from its packaging
- Inadvertent or spontaneous activation of a safety release
- Spontaneous activation from sideways force to remove the safety release
- User error
Congressional Hearings
Mylan acquired EpiPen in 2007 from Merck and began steadily increasing the price. In 2009, the wholesale price for an EpiPen two-pack was $103.50. By May 2016, that price had skyrocketed to over $600.
This sparked outrage from both the public and lawmakers, leading to a September 2016 congressional hearing with the then-CEO of Mylan, Heather Bresch.
- Rising research and development costs.
- The U.S. health care system causing some of the cost increases because insurance companies and pharmacy benefit managers pocket half of EpiPen’s wholesale price.
- Mylan needing to raise the price of EpiPens to cover the high costs of its EpiPen4Schools charity program.
After this hearing, Mylan offered patients “savings cards” to cover up to $300 in copays for EpiPen purchases. However, patients on Medicaid were ineligible for these cards, and people who used them often saw their health insurance premiums rise.
EpiPen Lawsuits and Class Actions
Mylan has faced EpiPen lawsuits and class action cases. In September 2016, the Department of Justice initiated legal proceedings against Mylan for misclassifying EpiPen as a generic drug to minimize the financial burden of its Medicaid rebates.
Manufacturers of generic medications owe less in rebates to Medicaid than brand-name medications. Mylan had to pay a $465 million settlement and reclassify EpiPen as a brand-name medication.
Price Gouging Litigation
In 2019, Mylan merged with a Pfizer subsidiary called Upjohn to create a new company, Viatris. In July 2022, Viatris settled a class action price gouging lawsuit for $264 million. The lawsuit claimed that Mylan and Pfizer maintained a monopoly by delaying patents and preventing generic competition.
These drugmakers allegedly struck an illegal deal with Teva Pharmaceuticals in which Teva would delay its generic version of the EpiPen if Mylan delayed its generic version of a Teva medication. This allowed both companies to increase product prices without worrying about being undercut by competitors.
In January 2025, Viatris agreed to pay $73.5 million to settle a related class action antitrust lawsuit. In this lawsuit, drug wholesalers accused Mylan of overcharging them for EpiPens by interfering with the release of generic versions.
Viatria agreed to pay, but did not admit to wrongdoing. The deadline to file a claim was May 29, 2025.
Product Liability Lawsuits
When medical devices contain defects, they can be the subject of product liability lawsuits. If EpiPens malfunction, they can cause serious injury, hospitalization or death, leading to litigation.
Patients and their families have filed lawsuits claiming that the 13 lots of recalled EpiPens failed to work correctly and provide the lifesaving medication, resulting in seven deaths.
Cost and Accessibility Issues
The cost of EpiPens has remained high despite lawsuits and other legal issues. It still costs over $600 out-of-pocket for a two-pack. Generic versions range from roughly $100 to $200 per pack when purchased with coupons.
For some people, epinephrine is a lifesaving medication that must be on hand at all times. Children may need to keep a pack at home and at school. This drives up the cost of an already expensive medication, and some may not be able to afford multiple packs.
Epinephrine may also expire before use. EpiPens only last for about a year, so they must be replaced regularly. This can be costly for anyone who needs them around, including schools, ambulances and health care facilities.
Several states have enacted legislation to limit the out-of-pocket costs for epinephrine auto-injectors. For example, Colorado has a state law that provides pharmacies with free generic EpiPens. However, this law is currently the target of litigation filed by drugmakers.
- Adrenaclick auto-injector
- AUVI-Q auto-injector
- Generic epinephrine auto-injectors
- Neffy nasal spray
- Symjepi prefilled syringe
What To Do if You’ve Been Harmed or Overcharged
Seek immediate medical care if you are injured by an EpiPen. Keep track of all medical records, photos and documents relating to your injury. You can file a report with the FDA’s MedWatch program.
If you or a loved one suffered serious injuries or death, you can speak with a product liability lawyer to review your options.
EpiPens are expensive. While using an expired one may save you money, you should try to keep an unexpired one on hand. EpiPens are for severe, life-threatening allergic reactions, and you want to be sure they will work properly.
If you think you were overcharged for an EpiPen, find out if your state has legislation to limit the cost of the device. You can also file an appeal with your insurance company.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




