Paragard Removal
Paragard removal is an outpatient procedure and typically takes a few minutes in a health care provider’s office. Common removal side effects are mild and include cramping and light bleeding. Rarely, the IUD may break upon removal, requiring surgery.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by Wilson & Peterson LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: March 7, 2025
- Est. Read Time: 3 min read
Paragard is a popular choice for women who want long-term, non-hormonal birth control. If a woman wishes to conceive, wants to change birth control methods, needs a new Paragard or has device side effects, she may choose to have the IUD removed.
Under the Affordable Care Act, IUD removal — including Paragard removal — is covered under most insurance plans at little to no cost. Patients should confirm this with their insurance company.
Preparing for Removal
The IUD can be removed any time during a woman’s menstrual cycle. Removal of an IUD is usually less painful and takes less time than insertion.
It’s common to have some cramping and bleeding after removal. Women may consider taking ibuprofen, acetaminophen or other over-the-counter pain reliever prior to arriving at the clinic or doctor’s office.
Taking a bath or shower before removal is also a good idea.
How Is Paragard Removed?
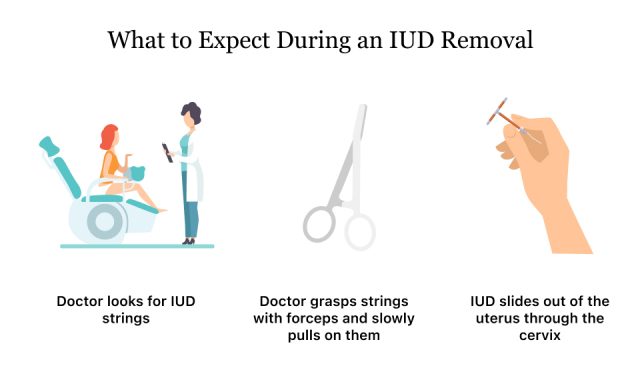
Women shouldn’t try to remove Paragard on their own, and a health care provider should remove it. Removal is a quick and easy procedure that doesn’t usually require surgery or anesthesia.
IUD removal is much like having a pelvic exam or IUD insertion. The patient will lie on her back with her feet on foot rests. The provider will use a speculum to hold the vagina open. He or she will cleanse the area, locate the IUD strings and gently pull. The Paragard’s arms will fold up upon removal and it will slide out of the uterus.
For some women, Paragard removal may be painful. It’s common to have light bleeding and cramping after removal. These symptoms may last a few minutes to several days after the procedure.
Patients can go home after waiting a few minutes at the provider’s office. Make sure to ask questions about what to expect before going home.
Paragard Removal Complications
In rare instances, Paragard may break during removal. When this happens, pieces may be difficult to remove. The device may also embed in surrounding tissues or organs.
According to Paragard prescribing information, “analgesia, paracervical anesthesia, cervical dilation, alligator forceps or other grasping instrument, or hysteroscopy may assist in removing an embedded Paragard.”
Dozens of women have filed Paragard lawsuits against the IUD’s maker after Paragard breakage caused complications such as infertility, organ damage or needing to have surgery to remove the device.
A 2022 research study identified 4,144 breakage reports for copper versus 2,140 for hormonal IUDs. Among the 170,215 adverse events reported, breaks were disproportionately higher for copper (9.6%) versus hormonal (1.7%) IUDs.
Side Effects
Most Paragard removal side effects are mild and last only a few minutes or days. Rarely, more serious side effects such as seizures can occur.
It can take up to three months for a woman’s period to return to normal after IUD removal, according to Nationwide Children’s Hospital.
- Dizziness
- Nausea
- Slow heart rate
- Cramping
- Light spotting
- Late or irregular periods
Call your doctor right away if you have heavier than usual bleeding, severe cramping that isn’t relieved by over-the-counter pain medications, bad smelling discharge, painful intercourse or a fever over 101 degrees.
Pregnancy After Removal
Paragard’s prescribing information says women may become pregnant almost immediately after IUD removal. Several studies have found using hormonal or copper IUDs doesn’t impair fertility after removal.
One 2018 review of studies by Tadele Girum and Abebaw Wasie in Contraception and Reproductive Medicine found that 83 percent of women got pregnant within one year of stopping contraception, including IUDs.
A woman’s age and health influence how soon she may conceive after stopping an IUD.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.






