Essure Side Effects
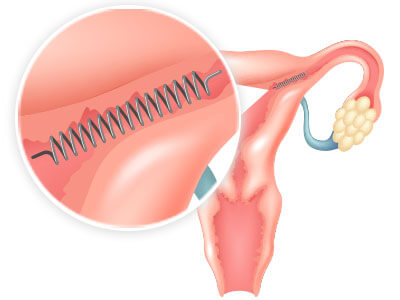
Commonly reported Essure side effects include pain near the implantation area and vaginal bleeding. More severe side effects include internal bleeding and coil migration. When implanted, Essure can build up scar tissue in the fallopian tubes, making pregnancy unlikely.
Article Continues Below
Board-certified physicians medically review Drugwatch.com content to ensure its accuracy and quality.
Drugwatch.com partners with Physicians’ Review Network Inc. to enlist specialists. PRN is a nationally recognized leader in providing independent medical reviews.
Reviewer specialties include internal medicine, gastroenterology, oncology, orthopedic surgery and psychiatry.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Short-Term Side Effects
- Pain at the implantation site, lower back pain and vaginal bleeding
- Potential Long-Term Side Effects
- Coil migration and organ perforation
- Blackbox Warning
- Patients have reported uterine or fallopian tube perforation, improper placement in the abdominal or pelvic cavity, persistent pain and potential for allergic or hypersensitivity reactions. Device removal requires surgery.
Latest Essure Side Effects Information
As of Dec. 31, 2019, all facilities with unused Essure units should have returned the units to Bayer. They are no longer available for implantation. Although Essure is no longer distributed in the United States, the U.S. Food and Drug Administration is committed to collecting long-term safety information on women who have received the device.
As of Dec. 31, 2023, the FDA had received 57,802 reportable events related to the Essure birth control implant since its approval in 2002. These events included 57,099 reports of serious injuries, 588 reports of malfunctions and 115 reports of deaths.
Short-Term Side Effects of Essure Implantation
Patients have reported both immediate and delayed short-term side effects, such as pain and vaginal bleeding, at various stages of the product’s implantation. The exact side effects experienced vary from patient to patient.
- Cramping
- Dizziness or lightheadedness
- Nausea
- Vaginal bleeding
- Vomiting
There were also risks specific to the implantation procedure. For instance, a piece could break off the device during implantation, necessitating the removal of the broken pieces.
Additionally, as with any medical device, allergic reactions to the materials might occur.
“A lot of women had severe allergic reactions to the device — about 20% of the population is allergic to nickel and not aware of it,” Holly Ennis, an Essure lawyer and partner at Ennis & Ennis, told Drugwatch.
In some cases, patients required anesthesia during the implantation. Anesthesia has its own set of side effects, including temporary nausea, headache, muscle twitching and dizziness.
Essure Confirmation Test
The reported side effects of the Essure Confirmation Test were similar to those reported during the implantation procedure.
- Cramping
- Dizziness
- Fainting
- Nausea and vomiting
- Spotting
Doctors would perform the confirmation test on a patient a few months after receiving an Essure implant to ensure the device was working properly. To complete the Essure Confirmation Test, a health care provider performed a transvaginal ultrasound (TVU) or a vaginal X-ray exam.
However, patients who underwent immunosuppressive therapies such as corticosteroid treatment or chemotherapy were discouraged from having a TVU performed.
Because Essure works by scarring the fallopian tubes, there was a high chance for people with compromised immune systems to have a delayed scarring reaction. Because TVU cannot detect scar tissue to confirm the device is working, doctors did not recommend this procedure for patients with immunocompromised systems.
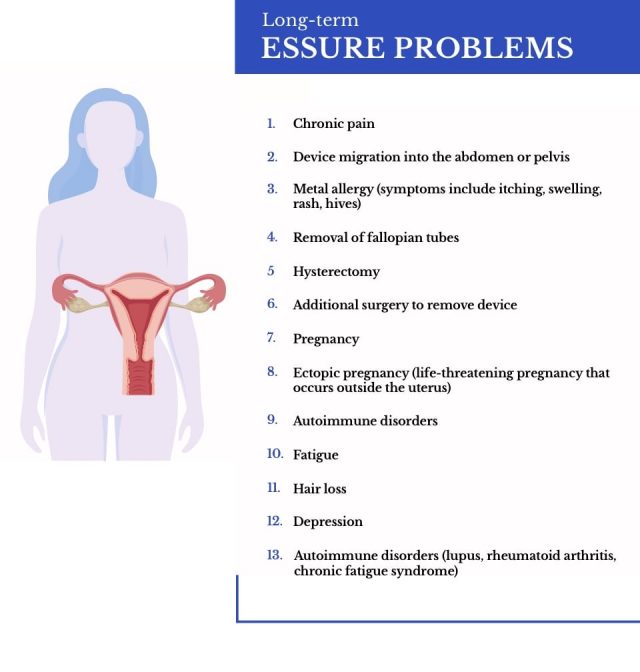
Long-Term Effects of Essure
Long-term adverse effects included pain, heavier period flows, perforation, headache and fatigue. Thousands of reports also mentioned movement or device dislocation. Over 1,100 women reported that the device did not meet their expectations due to malfunction or unexpected pregnancy. From 2017-2022, the majority of reports regarding Essure submitted to the FDA mentioned device removal.
Amanda Chavarria had Essure implanted when she was 22. Shortly after the procedure, she began having pelvic pain and irregular menstrual cycles. Then the back pain, fatigue, rashes, swelling and migraines started. She also became depressed and went through an ectopic pregnancy.
“All these medical problems back-to-back, my family labeled me a hypochondriac and an attention seeker,” she told Drugwatch.
Additionally, as a permanent contraceptive device, people who have Essure implanted have a lower chance of getting pregnant than those who do not use any form of birth control.
However, clinical studies indicate that 8% of women who are implanted with the device may still be fertile. The odds of pregnancy decrease over time, as women who have had the device for over five years exhibit less than a 1% chance of getting pregnant.
Essure Pulled from Production
On December 31, 2019, the manufacturer Bayer officially pulled all Essure devices from the market. The company stopped selling Essure and issued instructions to hospitals and doctors’ offices regarding the return of, and compensation for, previously purchased devices. Bayer advised against implanting any more devices. Today, the device is no longer distributed or implanted in the United States.
While the FDA never officially recalled Essure, it continues to investigate the implant. On February 12, 2024, the administration conducted a search of the Manufacturer and User Facility Device Experience (MAUDE) seeking data on Bayer from the day of its approval back in 2002 through December 31, 2023.
Essure Implant Removal
If you have Essure and are experiencing side effects such as debilitating pain, or you don’t feel you can rely on the implant to prevent pregnancy, speak to your health care provider about removing it. Implant removal requires surgery, which has its own risks and benefits unique to each patient.
From 2017-2018, the FDA evaluated medical device reports regarding the removal of Essure. The administration wanted to understand why approximately 17,000 patients were considering the removal procedure which required surgery. The most common reasons for seeking device removal were pain, genital hemorrhage and device dislocation/expulsion.
Additionally, some patients with the implant reported a metal allergy or a suspected metal allergy. Providers often recommended these patients to have their device removed.
Current Essure users who are not experiencing adverse effects do not need to have the device removed. The FDA assures patients, “If you have been using Essure successfully to prevent pregnancy, you can and should continue to do so.”
Alternatives for Essure
While Essure is no longer an option, there are other IUD options for those seeking long-term or permanent birth control. Some IUDs, such as Mirena, Kyleena, Liletta and Skyla, are hormonal. Others, such as Paragard, are copper.
Like Essure, a medical practitioner inserts these devices through the vagina. Most IUDs need to be replaced every 5-10 years, depending on the brand. IUDs have many of the same risks as Essure, such as penetration of the uterine lining.
Tubal ligation, which blocks, clips or removes the fallopian tubes, is another form of lasting birth control. Because this is a more invasive procedure, you and your doctor should discuss any potential risks or relevant health factors related to this surgery.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


