Exactech Replacements
Exactech replacements include joint replacements for knees, hips and ankles. Surgeons use these devices to replace arthritic or damaged joints. Exactech issued a recall for its polyethylene hip, knee, shoulder and ankle implant inserts because defective packaging could cause early wear and revision surgery.
About Exactech Replacements
Although Exactech’s product line includes medical software, instruments and biologics, its main products are its shoulder, hip, knee and ankle replacement devices. The company manufactures joint replacements for primary surgery as well as revision surgery.
Its replacements are used in high-volume surgical centers such as the Hospital for Special Surgery in New York and Shands Hospital in Gainesville, Florida, where the company is headquartered.
However, over the years, the company has faced lawsuits related to knowingly selling defective products to government entities and allegedly engaging in illegal consulting agreements with doctors. And as of April 2025, there were 1,842 Exactech recall lawsuits pending in multidistrict litigation before the U.S. District Court for the Eastern District of New York.
Plaintiffs allege that the recalled joint implants failed prematurely, requiring revision surgery. So far, the device maker has recalled more than 600,000 knee, hip, shoulder and ankle prosthetics manufactured between 2004 and August 2021.
Exactech Hip Replacement
Exactech hip replacement models include Alteon, Novation, BIOLOXdelta, AcuMatch and MCS. The company manufactures primary hip replacement and revision surgery hip replacement systems. Some studies have shown that Exactech’s hip implants have a satisfactory survival rate of about five years, meaning a lower chance that the implant would have to be replaced at five years.
One 2022 study published in Hip Arthroplasty found that the Novation Element Stem had at least a 92.4% survival rate at five years, but study authors stress that more long-term studies are needed to assess the implant’s performance over time.
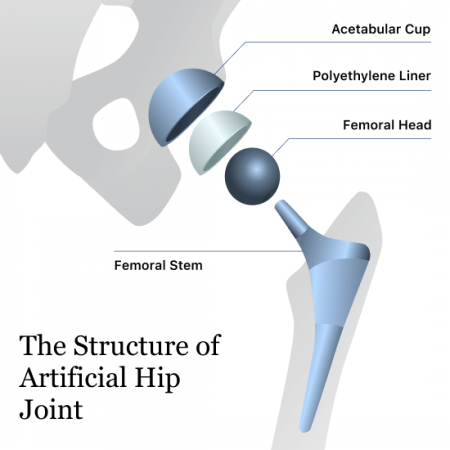
However, some researchers have noted that patients with Exactech Connexion GXL polyethylene liners may have a higher rate of osteolysis, or bone tissue damage. These plastic liners go between the acetabular cup that fits into the hip socket and the ball attached to a stem in the thigh. The liners help the ball rotate against the cup, mimicking a natural hip joint.
Beginning in June 2021, Exactech recalled the polyethylene liners, including the GXL liner, in nearly 40,000 of its Novation, AcuMatch and MCS hip devices because of the potential for early failure and above-average rates of revision surgery. According to the company, the recalled hip liners were packaged in defective vacuum-sealed bags that allowed oxidation of the parts.
Exactech Knee Replacement
Exactech knee replacement models include Truliant and Optetrak. One 2021 study published in The Knee found that Optetrak Logic and Truliant single-option knee implants improved the fit of the femoral component in Asian patients over older designs from other companies such as Anthem, Attune and Persona.
The company has also improved its Optetrak Logic knee design, which resulted in a lower rate of revision surgery and patients complaining of pain. However, the new design had a slightly higher rate of revision surgery for osteolysis, according to a 2020 study published in HSS Journal: The Musculoskeletal Journal of Hospital for Special Surgery.
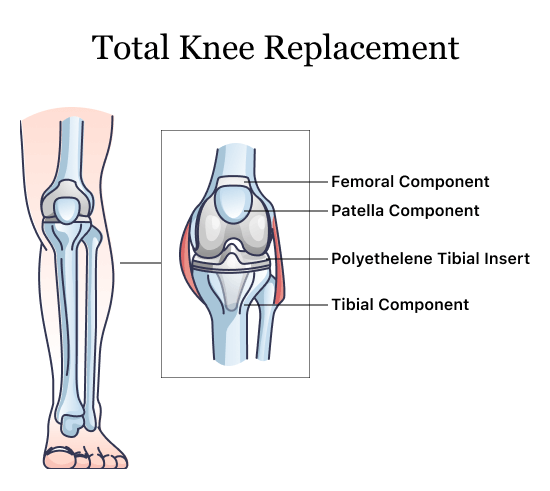
A 2021 study published in the Journal of Knee Surgery found the Optetrak Logic posterior-stabilized (PS) total knee arthroplasty (TKA) femoral component was associated with loosening in less than five years compared to other brands. The authors said the “earlier failure rate with this implant is of concern.”
In August 2021, Exactech initiated a recall for all its ultra-high-molecular-weight polyethylene inserts in knee replacements. The recalled inserts were packaged in out-of-specification bags that could allow oxygen to get to the insert and cause them to degrade and lead to early implant failure.
The company has recalled 489,498 of its Optetrak Logic, Truliant and Arthrofocus knee implants manufactured between 2004 and August 2021.
Exactech Knee Implant Patient Story
The husband of one Exactech knee replacement recipient, who is being identified as NG to protect her identity, told Drugwatch that NG had problems with her knee implant about six or seven years after her original surgery.
“About the time that the knee pain was getting worse, we were notified from Exactech about the recall due to the improperly stored pad prior to the original surgery. My wife had approximately two-thirds of the upper knee replaced through revision surgery,” he said.
Exactech Ankle Replacement
The Vantage Total Ankle System and Vantage PSI are Exactech’s main ankle replacement products. They are designed to mimic a patient’s natural anatomy. In one 2021 study published in the Journal of Clinical Medicine, researchers found the Vantage Ankle System design reduced prosthesis strain and failure.
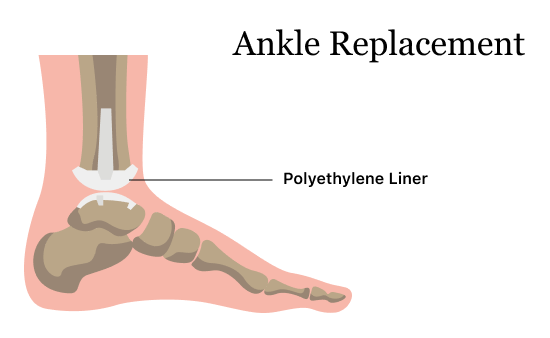
In August 2021, Exactech issued a recall for the polyethylene liner for all ankle replacements, and they later updated the recall in February 2022. The recalled polyethylene plastic insert mimics cartilage in the ankle and fits between the talar component and the tibial component.
This ankle liner recall coincides with the recall for liners also used in knee replacements and is also because of faulty packaging that may allow liners to degrade. Not all patients may have problems with their devices, and Exactech doesn’t recommend replacing ankle devices in people who aren’t having issues with their implants.
Exactech Recalls
The initial August 2021/February 2022 Exactech recall applies to hip, knee and ankle replacement inserts manufactured from 2004 to August 2021. These recalls were for defective packaging that could allow oxygen inside of vacuum-sealed bags and cause plastic liners in the devices to degrade prematurely.
In March 2023, the FDA released a Medical Device Safety Communication reminding medical providers and the public that many of Exactech’s hip, ankle and knee replacement devices manufactured between 2004 and August 2021 were packaged in defective packaging.
In January 2024, the FDA released a Safety Communication warning to the public about Exactech’s Equinoxe Shoulder System, which was also packaged in defective packaging from 2004 to August 2021. In March 2024, Exactech initiated a voluntary recall of its shoulder replacement devices.
- Check your implant’s ankle or knee implant’s serial number on Exactech’s recall page. Typically, this serial number will be in your medical records. If you aren’t sure where it is, contact the surgeon who implanted your device.
- If you are having problems such as pain, swelling or difficulty moving the joint, contact your doctor right away.
- Talk to your surgeon if you have a recalled implant to discuss plans for monitoring your implant even if you aren’t experiencing problems.
- Your surgeon may call you if you have a recalled implant, and they may request you come in for a follow-up appointment even if you aren’t having problems. Osteolysis might be present even if there are no symptoms.
Exactech recommends contacting them to file a claim if you have a recalled implant and your doctor contacts you for a follow-up. They will address recall-related out-of-pocket expenses.
Warning Signs of Recalled Implant Complications
Not everyone with a recalled Exactech implant will suffer complications or have symptoms.
“It’s important that patients recognize the difference between the normal healing process and symptoms that might signal a problem with the implant. Be sure to ask your surgeon what those symptoms might feel like,” Dr. Thomas Pontinen, a double board-certified pain management expert and surgeon, told Drugwatch.
The FDA and Exactech don’t recommend having surgery unless you have issues with your implant. Pain after surgery is a normal part of healing, but excessive pain that doesn’t go away could signal a problem that your device has failed or needs to be replaced.
“Experiencing a little bit of discomfort is normal post-implant surgery but prolonged or worsening pain, especially during simple daily activities, is a red flag for potential issues."
Dr. Pontinen has extensive experience with patients who suffer pain from hip and knee replacements. He shared that some of his patients who have had hip or knee implants have trouble doing daily activities like climbing stairs because of pain.
Potential complications of a failed implant include:
- Pain
- Stiffness
- Loosening
- Device failure (and revision surgery)
- Limited range of motion
- Osteolysis (bone loss)
“These symptoms can be truly detrimental to someone’s quality of life. Experiencing a little bit of discomfort is normal post-implant surgery but prolonged or worsening pain, especially during simple daily activities, is a red flag for potential issues,” he said.
Exactech Replacement Lawsuits
Some people have chosen to file Exactech lawsuits for any expenses incurred, additional surgeries and any pain and suffering related to their implant. Plaintiffs in lawsuits claim Exactech manufactured a defective product and failed to warn the public about the risks of early failure and revision surgery.
If you are interested in filing a knee or hip replacement lawsuit, speak to a product liability lawyer. Even if you’ve received some reimbursements from Exactech through a Broadspire claim, that claim won’t pay for pain and suffering and other costs. However, a lawsuit may qualify you for more compensation.
Daniel Nigh, a mass torts and product liability attorney, says people who have received notice of the recall of their Exactech implant should contact an attorney immediately — regardless of whether they have undergone revision surgery yet.
“It also allows the attorneys who are actively litigating the cases to have insight and make sure that their statute of limitations is protected, even if they haven’t had a revision yet,” Nigh said.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.