Depo-Provera:
A Convenient Shot, But at What Cost to Women’s Health?
Depo-Provera, a widely used birth control shot, has been praised for its convenience and affordability. But mounting research and alarming personal stories reveal a darker side to the shot — serious health risks and potentially life-altering meningioma tumors. Despite growing evidence, warnings about the tumor risk remain absent from the U.S. product label. This investigation explores the true price of Depo-Provera, its disproportionate danger to marginalized communities and the ongoing legal battles seeking justice for those harmed.
Drugwatch.com content is legally reviewed for accuracy and quality.
Examples of trusted legal reviewers include qualified mass torts lawyers and certified paralegals.
Drugwatch.com writers gather lawsuit information by studying court records, watching lawsuit proceedings and speaking with experienced attorneys.
The Untold Stories Behind America’s Popular Birth Control Injection
Tina Thomas was cleaning out her closet when her phone rang. She answered, but no one was on the other end of the line.
She hung up to move on with her day, but the phone quickly rang again. She answered it but still heard no one.
It wasn’t until the third call that Thomas held the phone up to her other ear.
“I switched ears, and the person on the other end said, ‘You kept hanging up on me. I kept saying hello. Didn’t you hear me?’” Thomas told Drugwatch. “I didn’t realize that I was losing hearing in my ear.”

That alarming discovery eventually led to the diagnosis of a meningioma, a tumor that affects the membranes surrounding the brain. It would drastically alter Thomas’ life.
“The tumor was lying on the nerves that controlled my hearing, so I have permanent hearing loss,” she said.
Thomas underwent an invasive procedure to remove the tumor in an effort to keep the situation from worsening. But the meningioma later returned, this time impacting her vocal cords. After undergoing another surgery, she was left with a raspy voice.
“That meningioma has really scarred my life,” she said.
It wasn’t until late last year that Thomas learned of a potential cause.
“A couple months ago, I was watching TV and a commercial came on about the meningiomas and how they’re linked to the Depo-Provera shot,” she said.
Thomas — and many women like her — didn’t realize that her life-altering condition may have been caused by Depo-Provera. Until her diagnosis, she had been given no reason to doubt her birth control.
While the birth control shot had been marketed as safe and effective, mounting evidence links the drug to the development of meningioma tumors.
“You mean to tell me that they knew about this and they’re still giving these shots to women? They just did not value our lives at all.”
But Depo remains available across the U.S. with no mention of a meningioma threat from manufacturer Pfizer or the Food and Drug Administration, despite warnings listed in other countries.
When Thomas first learned that a connection may exist between her birth control and the condition that devastated her life, she was angry.
“You mean to tell me that they knew about this and they’re still giving these shots to women?” Thomas said. “They just did not value our lives at all.”

An ‘Interesting’ Risk-Benefit Calculus
It didn’t take long for Depo-Provera to rocket in popularity after it was introduced in 1992. The drug was marketed as a simple injection that only required four shots per year to be highly effective.
But beneath its accessibility lies an alarming concern. A 2024 study published in the BMJ revealed that long-term users of Depo-Provera may be at more than five times greater risk of developing a meningioma. The study analyzed the cases of more than 100,000 women.
Dazhi Liu, an oncology clinical pharmacy specialist, told Drugwatch that the study helped identify meningioma risks for progestin-based medications like Depo-Provera.
An under-review study by the University of British Columbia links meningiomas to long-term Depo-Provera use. Researchers found that using the birth control shot for more than a year could triple your risk of developing a meningioma.
“Given that there are so many different contraceptives to choose from, I think it makes the risk-benefit calculus more interesting,” Dr. Mahyar Etminan, the lead researcher of the new study, told Drugwatch.
Etminan pointed out that there are many other birth control options to consider that don’t carry the same level of risk for meningiomas or other serious conditions.

As Other Nations Warned of Risk, FDA Blocked a US Label Update
It didn’t take long for other parts of the world to leap into action after meningiomas were tied to Depo-Provera.
Just days after the release of the BMJ study, the Guardian reported that Pfizer said, “We are aware of this potential risk associated with long-term use of progestogens and, in collaboration with regulatory agencies, are in the process of updating product labels and patient information leaflets with appropriate wording.”
The European Medicines Agency went on to recommend that the meningioma risk be added to Depo warning labels in the European Union. They suggested that patients on high doses of the drug be monitored for meningioma symptoms.
The FDA, meanwhile, didn’t respond in kind. Despite meningioma risks being listed on Depo labels in Canada, the U.K. and the EU, users in the U.S. have still not been officially warned of the potential connection.
In fact, Pfizer claims that the FDA actively blocked the company from warning consumers.
The drug manufacturer stated in court documents that it submitted label changes to the FDA at the same time that it did so in Europe. The updated language would have included an alert on meningiomas and a warning to stop taking the drug if users suspected a tumor.
But the FDA rejected that label update. The agency decided that the study data didn’t justify a warning to U.S. consumers, even as European nations alerted their citizens.
Women Chose Depo for Its Affordability and Availability
Depo-Provera is cheap, available almost everywhere and doesn’t require a prescription from a physician in many parts of the country.
Drugwatch surveyed 650 women between the ages of 18 and 70 and found that about 20% have used a birth control injection like Depo.
According to Planned Parenthood, the shot is free or low-cost with Medicaid, other government programs and many health insurance plans. For millions of women, it’s an easy and accessible option.
Thomas received her first Depo shots from Planned Parenthood after trying several different birth control methods.
“I wanted to protect myself however I could,” she said.
KFF, an independent organization for health policy research, says Medicaid and Affordable Care Act plans cover the shot. These insurance options cover a doctor’s visit, side effect management and eventually stopping the shot. For many women, there’s seemingly little downside to trying out the birth control shot.
“It sounded too good to be true. But we believed it.”
Sixteen states, including major population centers like California and Illinois, allow pharmacies to prescribe Depo-Provera without a doctor’s approval.
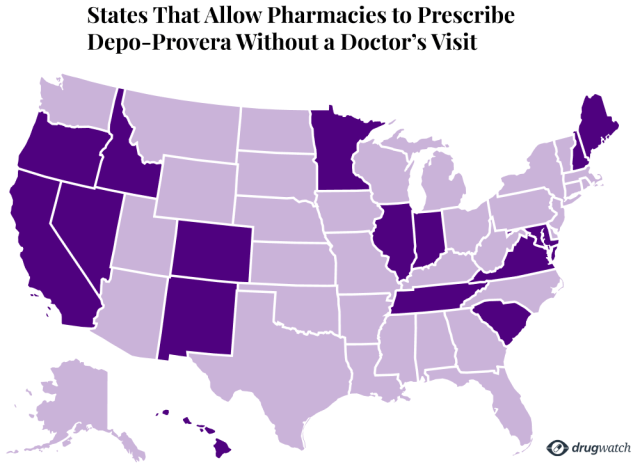
- California
- Colorado
- Hawaii
- Idaho
- Illinois
- Indiana
- Maryland
- Maine
- Minnesota
- Nevada
- New Mexico
- New Hampshire
- Oregon
- South Carolina
- Tennessee
- Virginia
Depo-Provera use is widespread and isn’t limited to those seeking birth control. Depo is also sometimes recommended by doctors as a treatment option for other health conditions.
Edie R. began taking the Depo shot decades ago to help manage Asherman’s Syndrome, a condition where scar tissue forms inside the uterus.
“When I went to my new gynecologist, she told me about this wonderful new drug,” Edie told Drugwatch. “She said the only side effect is weight gain … it sounded too good to be true. But we believed it.”
Edie R. was hopeful that she would receive a needed boost to manage her existing condition.
Instead, her life devolved into a daily battle against debilitating symptoms and constant seizures. She eventually needed surgery to remove the meningioma that was causing adverse effects.

Meningioma: A High Price to Pay
The low cost of Depo-Provera has been touted as one of its major benefits, bringing affordable birth control to millions of women.
But some users say the low-cost shot left a crippling financial impact.
While meningiomas are generally benign, they often need to be surgically removed to prevent them from pressing on the delicate structures of the brain. In many cases, this isn’t a medical problem that can be ignored.
According to Liu, patients may have headaches or more serious issues like memory loss, confusion and even seizures.
“In some patients, if the tumor is near the optic nerve, [it] can also affect vision. Or if it’s close to your auditory structure, it can affect hearing as well,” she said.
Resection, or removal, of the tumor is often necessary to correct those concerns.
Meningiomas are often removed through a craniotomy. According to CBS News, this is one of the most expensive medical procedures in the U.S., with an average cost of $66,935 before insurance.
“That meningioma has really scarred my life.”
Even for those with insurance, out-of-pocket costs can easily exceed $1,000. And the surgery may only be the beginning of the expenses.
“For many individuals, the financial toll can be significant,” Brendan Smith, a partner with Simmons Hanly Conroy working on Depo-Provera litigation, told Drugwatch. “Meningiomas can lead to surgery, hospitalization, ongoing neurological care and potential long-term disability, making medical bills and lost wages overwhelming.”
For Edie R., the medical requirements piled up after her surgery. She needed speech, occupational and physical therapy to aid in her recovery.
The diagnosis also took a toll on her career. She was forced to give up her job as an accountant, a role she had held since the 1980s.
“I had to quit my job. I couldn’t do it anymore,” she said. “The financial devastation has been enormous in lost earnings. I tried to go back to work after my surgery. I started with temp jobs, and I went backwards. It was too hard on me. It drained me.”
Thomas needed multiple surgeries to remove meningiomas after her tumor came back. She also needed radiation treatment in addition to those procedures, all complicated by the fact that she couldn’t work for months.
“It was really tough,” she said. “But my family, they stood in for me and they helped me out.”

Unseen Costs: The Emotional Toll of a Meningioma Diagnosis
The financial and medical costs of dealing with a meningioma can be severe, but the emotional cost can be even more challenging.
Throughout her entire life, Thomas loved to sing. She would sing in competitions, pageants and other events.
But when the meningioma came back, her voice became hoarse. The tumor had grown in a different shape and was now lying on her vocal cords. Even following treatment, her voice remained permanently damaged and raspy, taking away one of the joys of her life for good.
“I would sing whether I was happy, whether I was sad. It was something that I loved to do,” she said. “There have been so many things that have been taken away from me.”
After Edie R. had a craniotomy to remove her meningioma, the seizures that had impacted her daily for years finally subsided. But nearly every other symptom that she had worsened after the procedure.
“The dizziness got worse, the cognitive problems got worse, all the symptoms I had before were far worse,” she said. “Now I could no longer ride in a car without taking Meclizine.”
Edie was left disabled and unable to drive. She also lost her ability to sew, which had been an important pastime throughout her life. Following the impacts of the meningioma, she couldn’t safely maneuver the machine.
“I still can’t use a sewing machine without sewing through my front fingernail,” she said. “I stitched through that fingernail three times before I gave up on it and decided I just can’t. So, I lost that. A lifelong hobby.”

Gender Bias Compounds Emotional and Financial Costs
Some women have faced an additional obstacle before they could even receive treatment for a meningioma — getting their doctors to believe them.
Thomas and Edie said that they had to constantly advocate for themselves for their concerns to be taken seriously, with their symptoms often dismissed.
It could take months — or even years — after first experiencing symptoms and seeking help to receive a diagnosis. For some women, it takes multiple trips to their doctors, emergency rooms and specialists.
“There is a perception that women’s problems are less significant,” Dr. Luke Twelves, a general practitioner and VP of Medical at Lindus Health, told Drugwatch. “And a lot of patients come in expecting to be dismissed whether they’re seeing a male doctor or a female doctor.”
Even after realizing she was losing hearing in one of her ears, Thomas still didn’t receive the help she needed. Her doctor sent her to a specialist. After playing piercing sounds that Thomas could still hear, the specialist determined nothing was wrong.
She had already been turned away empty-handed following multiple doctor’s trips for severe headaches. She finally decided to visit the ER again, but this time at a different hospital. It was there that she was finally diagnosed with a meningioma.
“It was extremely frustrating because I knew something was wrong,” she said. “You know your body and there’s no way that I’m taking trips to the emergency room just for the fun of it.”
According to Duke Health, one in five women say that a health care provider has ignored or dismissed their symptoms at some point.
Delayed care for women can come at a serious cost. Some conditions may go untreated, leading to poorer outcomes. It can also mean rising expenses, with more doctor’s visits and time off work.
Thomas spent an entire year going back and forth between doctors before she was finally diagnosed with a meningioma.
These delays may partly be due to historical gender bias in medicine. It wasn’t until the early 1990s that women were generally invited to participate in clinical trials, meaning much of the existing research up to that point focused on men.
As an example, Twelves pointed to cardiovascular disease — a major health issue in women.
“Because all the research has been done on how cardiovascular disease presents in men, men tend to get better care,” he said. “It’s recognized earlier. They’re treated earlier, they’re managed earlier.”

Depo’s Disproportionate Danger to Vulnerable Populations
The health, financial and emotional consequences of Depo-Provera may be felt most heavily by communities already facing social or economic challenges, including women of color, people who have completed lower levels of education and young women.
Women of Color
Depo-Provera has long been popular in minority communities, with Black women being the most likely to use the injectable birth control option.
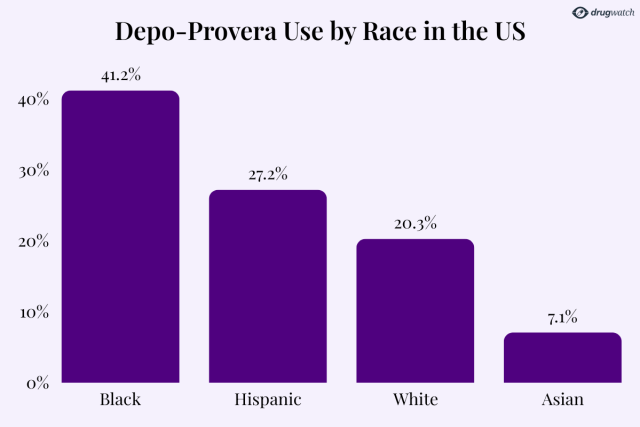
A CDC report revealed that 41.2% of Black women surveyed had used Depo-Provera, far exceeding usage rates among Hispanic (27.2%) and white women (20.3%).
According to the book Black Women and International Law, 84% of Depo-Provera users in the U.S. are Black.
The Depo shot was the second most-used birth control option among Black women in the U.S., behind only birth control pills. Its use sits far ahead of other options like IUDs or emergency contraception.
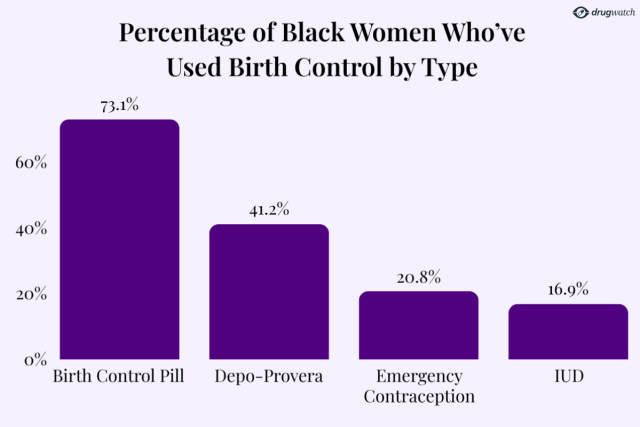
According to KFF, one in three Black women say that they have had negative health care experiences that worsened their health or reduced their likelihood of seeking medical care.
Women Without a High School Diploma or GED
The CDC found that nearly 40% of women without a high school diploma had used Depo-Provera, compared to just 12.7% of women with a bachelor’s degree or higher.
The rate of Depo use steadily decreases as education levels rise.
“Ever use of Depo-Provera was roughly three times as common among women without a high school diploma or GED compared with those with a bachelor’s degree or higher,” the CDC reported.
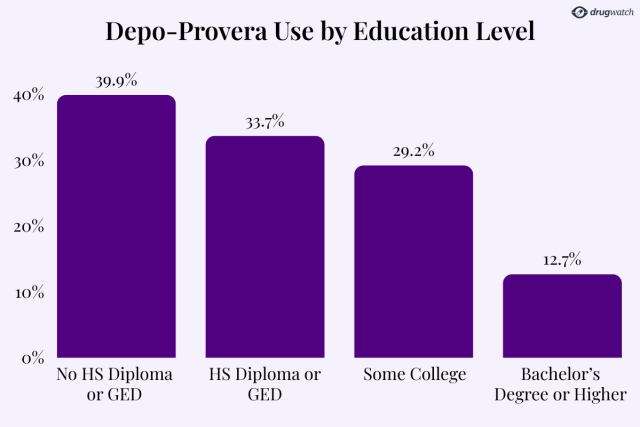
By education level, the least likely group of sexually active women to use the Depo shot were those who had earned a bachelor’s degree or higher.
Younger Women
Women who use Depo-Provera tend to be younger. Black Women and International Law noted that a third of Depo users in the U.S. are under the age of 19.
In addition to the growing meningioma concerns, younger users could also be susceptible to bone loss issues. Depo-Provera includes warnings that the use of the drug may lead to a decrease in bone density and could cause osteoporosis.
“The longer you use Depo-Provera CI, the greater your loss of calcium from your bones,” the drug’s warning states. “Your bones may not recover completely when you stop using Depo-Provera CI.”
The label includes no mention of a meningioma risk.

Lawsuits Seek Justice for Women Who Are Impacted
More than 100 women who’ve used Depo-Provera and been impacted by meningioma have reached out to Drugwatch for legal help.
Many Depo-Provera lawsuits have already been filed claiming that Pfizer failed to warn the drug’s users of the risk of serious health conditions.
“There have been so many things that have been taken away from me.”
Earlier this year, the U.S. Judicial Panel on Multidistrict Litigation consolidated 78 Depo-Provera lawsuits before one judge in Florida. That number has since ballooned to 130 active cases. This key step in emerging litigation could lead to quicker results for plaintiffs.
“Many of the plaintiffs report feeling betrayed and frustrated,” Smith said. “They trusted Depo-Provera for its convenience and effectiveness, believing it was a safe option.”
Edie R. says that she realized that there may be a connection when she was first diagnosed with a meningioma years ago. She began researching the tumor and learned that it often has progesterone receptors, meaning meningiomas may be tied to exposure to the hormone.
Depo-Provera contains progestin, a synthetic version of progesterone.
“I wondered. I was still taking that freaking shot,” she said. “I’d already tied it enough in my head that I told both my daughters, ‘Don’t take that.’ They both would have considered it, and they both knew what happened to me.”
The lack of warnings to U.S. consumers will likely be a key topic in the litigation. Unlike the U.S., the U.K. and EU have updated Depo warning labels. The Canadian version of the drug has referenced meningioma tumors for a decade.
“This can certainly be a central point of contention,” Smith said. “Courts will look at the timing of those label updates and whether Pfizer had evidence that should have prompted earlier warnings in the United States.”
As litigation gets underway, plaintiffs are hopeful that they will see justice, even as they work to pick up the pieces of their lives and move past what they have been through.
“You have to have a positive attitude to get through life. If you don’t, you’re just going to lay down and die.” Edie R. said. “So, I’m OK. But I don’t know if anyone else that had gone through what I’ve gone through would be OK.”
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.