Gadolinium Side Effects
The most common gadolinium side effects are injection site pain, itching and nausea. These are typically mild. These contrasting agents carry a boxed warning for gadolinium retention and nephrogenic systemic fibrosis, a multiorgan disorder characterized by thickening skin and organ tissue.
Article Continues Below
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Common Side Effects
- Headache, nausea, dizziness and injection site reactions
- Serious Side Effects
- Allergic reactions and gadolinium toxicity
- Boxed Warning
- Gadolinium-based contrast agents (GBCAs) increase the risk of nephrogenic systemic fibrosis (NSF) in people with serious kidney problems.
Most Recent Gadolinium Side Effects Information
As of June 30, 2024, the most common gadolinium side effects reported to the U.S. Food and Drug Administration were nephrogenic systemic fibrosis (NSF), pain and joint stiffness. Those three side effects account for over 91% of all reports to the FDA.
- Nephrogenic Systemic Fibrosis: 39.25%
- Pain: 29.03%
- Joint Stiffness: 23.13%
- All Other Side Effects: 8.59%
The FDA also considers 91% of reported gadolinium side effects to be serious.
| FDA Adverse Event Reports for Gadolinium Side Effects | |
|---|---|
| Total cases reported | 1,643 |
| Serious cases (including deaths) | 1,497 |
| Deaths | 197 |
Disclaimer: Reports sent to the FDA don’t necessarily mean the drug caused an adverse event. Consult a health care professional before stopping or changing medication.
Prescribing information for Magnevist (gadopentetate dimeglumine) shows that gadolinium-containing contrast agents are generally well-tolerated and most patients have either no adverse effects or mild, short-term symptoms.
Reporting adverse events to the FDA is voluntary, so the numbers may not reflect all cases of adverse events. These numbers indicate cases of serious side effects and not the typical patient experience.
Boxed Warning for Nephrogenic Systemic Fibrosis (NSF)
The FDA mandates that GBCAs have a boxed warning for nephrogenic systemic fibrosis. This disease hardens and thickens the skin and internal organs, potentially leading to death. Patients with an impaired ability to filter out drugs in their bodies are at an increased risk.
Patients with kidney problems have the highest risk of developing NSF. Patients with chronically reduced renal function should not receive GBCAs unless there is no way for an MRI to provide essential diagnostic information without contrast dye.
Common Gadolinium Side Effects
The most common side effects of gadolinium, headache and nausea, generally occur at rates below 5%. Many patients tolerate GBCAs well and have no adverse reactions.
- Dizziness
- Fatigue
- Headache
- Injection site pain
- Itching
- Nausea
- Pins and needles sensation
- Rash
- Restlessness
- Shortness of breath
- Vomiting
Most gadolinium-containing medicines have similar rates of side effects. For example, around 5% of patients in the clinical trials for Gadavist (gadobutrol) and Magnevist (gadopentetate dimeglumine) experienced adverse reactions, with headache being the most common.
Awareness of the side effect risks of GBCAs is low, which can complicate discussions between doctors and patients.
Serious Adverse Reactions
Serious adverse reactions to gadolinium-based contrast agents are rare, but patients should be aware of the risks of potentially fatal allergic reactions and gadolinium buildup within the body. This can cause nephrogenic systemic fibrosis, a multiorgan disorder involving debilitating or fatal thickening of the muscle, skin and internal organs.
- Acute kidney injury
- Chronic, severe kidney disease
- Nephrogenic systemic fibrosis (NSF)
- False positives for arterial stenosis (narrowing of the arteries)
Gadolinium deposition disease resembles NSF and can cause brain fog, abnormal skin sensations, headache, fatigue, insomnia or muscle twitches.
A common theory is that people with healthy kidneys who develop GDD may have a genetic abnormality that prevents their bodies from filtering out heavy metals.
Allergic Reactions to Gadolinium
In rare cases, patients may experience mild to severe allergic reactions to gadolinium. These may manifest in anaphylaxis or cardiovascular or respiratory symptoms. Most reactions occur in the first half hour after administration. Delayed reactions are rarer but can occur within hours or even a week after receiving a dose.
Severe hypersensitivity reactions can be fatal if untreated. Medical professionals should discuss a patient’s medical history before administering gadolinium to determine risk factors like allergies or asthma.
- Altered taste
- Anxiety
- Cough
- Flushing
- Hives
- Itching
- Nasal congestion
- Nausea
- Rash
- Sneezing
- Sweating
- Swelling of the face or eyes
- Vomiting
- Warm sensation
Serious symptoms of an allergic reaction include rapid heart rate, low blood pressure, feeling out of breath, swelling of the throat, narrowing of airways or slowed heart rate. Severe reactions like respiratory distress, convulsions, irregular heartbeats, anaphylaxis and cardiac arrest require immediate medical attention. Those with allergic reactions may want to consider alternatives.
Gadolinium Alternatives
High-risk patients and those worried about the possible side effects of gadolinium-based contrast agents may opt to get an MRI without the contrast. However, certain situations and diagnoses require contrast for the most accurate results.
Current research examining alternatives to reduce gadolinium exposure shows some promising results. A 2024 study in Nature explores the possibility of using diffusion-weighted imaging for fibroids.
An additional study published in the Journal of Molecular Imaging and Biology in April 2024 weighed the advantages of using an iron-based contrast agent in lymphatic imaging. Patients can talk to their doctor to discuss their options and weigh the risks with the benefits.
Gadolinium Retention
The FDA released a safety bulletin in December 2017 warning that gadolinium can remain in certain organs of the body for months or years. GBCAs can linger in bone and tissues of the brain, kidney, liver, skin or spleen.
Patients who receive numerous doses throughout their lifetime have a higher risk of retention. Some patients, especially ones with healthy kidneys, have filed gadolinium lawsuits blaming the material for causing serious injuries and health problems.
Additionally, there are two classes of GBCAs: linear agents and macrocyclic agents.
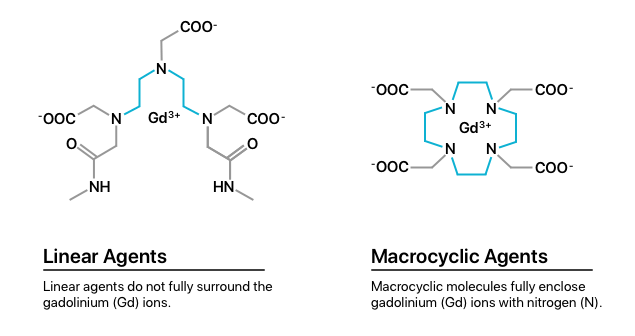
Multiple studies show that the brain holds onto more gadolinium particles from linear agents than macrocyclic agents. Patients who receive linear agents may be at higher risk for gadolinium retention or toxicity.
Case Study: Gena Norris’s Gadolinium NSF Side Effects
Gena Norris, actor Chuck Norris’s wife, experienced side effects after getting gadolinium for MRI scans, according to court documents. She claimed she felt like her whole body was burning and was in excessive pain. These problems wouldn’t go away, and she didn’t get a clear answer from her doctors about what was causing them, according to her lawsuit complaint.
Tissue Thickening and Mental Fog
Norris experienced burning pain and had difficulty moving her arm because of tissue thickening. She also felt mentally foggy. These symptoms were similar to those of nephrogenic systemic fibrosis (NSF), a rare condition linked to gadolinium retention. Even though the FDA says NSF typically affects people with kidney problems, Norris had normal kidney function.
Long-Term Effects and Recovery
Gena had a tough time getting better. She stayed at a clinic in Reno, Nev., for five months, getting IV treatments to remove gadolinium from her body.
Gadolinium Toxicity
While rare, gadolinium toxicity can develop in patients within hours or even years later if the patient’s body retains too much of the gadolinium. This results in symptoms ranging from mild to severe.
- Bone or joint pain
- Brain fog
- Burning pain
- Changes in vision or hearing
- Cognitive issues
- Diarrhea
- Difficulty breathing
- Headache
- Kidney damage
- Metallic taste
- Nausea
- Numbness
- Skin thickening or discoloration
- Tingling
- Vomiting
The risk of toxicity increases in patients who have multiple MRIs with contrast. Children, pregnant people, and people with inflammatory conditions or kidney problems also have a higher risk level.
Blood and urine screenings can test for gadolinium toxicity. If a patient screens positive for toxicity, doctors may prescribe chelating agents such as diethylenetriaminepentaacetic acid (DTPA). Chelating agents bind to heavy metals and can help your body filter out any remaining gadolinium.
However, these agents may cause mineral depletion, so they should only be used under the supervision of a health care provider.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


