How to Read a Drug Label
Drug labels for over-the-counter and prescription medicines provide vital information on safe and proper use. However, patients might have trouble finding the information they need. This guide offers tips from health experts to help you learn how to read drug labels.

Reading drug labels can help you ensure you are taking a medication correctly and inform you of possible side effects. Unfortunately, these labels can be challenging for the average person to understand.
If you find the information on your drug labels confusing, you are not alone.
In an interview with Drugwatch, Mireille Hobeika, who received her doctorate in pharmacy from the University of Saint Joseph in West Hartford, Connecticut, said, “Several studies have shown that patients often have difficulty in reading and understanding medication labels, which may lead to medication errors such as taking the wrong medication or the wrong dose.”
Understanding drug labels is particularly important for seniors and people with chronic illnesses. These groups typically have the greatest risk of medication errors or interactions because they tend to take multiple medications.
There are two kinds of drug labels:
- Over-the-counter drug labels: Also called Drug Facts, these labels include information like active ingredients, warnings and directions.
- Prescription drug labels: These may include many pages of safety information, such as pharmacy information sheets, medication guides and prescribing information.
Before you start taking a new medication, review the drug label with your doctor and pharmacist. Let them know about any health conditions you have and any medications and supplements you take.

Over-the-Counter Drug Facts
Over-the-counter, or OTC, drugs are medications that don’t require a prescription. Manufacturers print drug labels called Drug Facts directly on OTC drug product packages.
The Food and Drug Administration classifies any product with a substance intended for diagnosis, cure, treatment, prevention or mitigation of a disease as a drug. This includes products like fluoride toothpaste and anti-dandruff shampoo.
If you are unsure whether a product is an OTC drug, check the packaging for a Drug Facts label.

Four out of five adults in the U.S. commonly take OTC drugs, according to an article published in the Pharmacy Times.
The most popular OTC drugs in 2016 included pain relievers, heartburn drugs like Prilosec and Nexium, upper respiratory drugs and toothpaste.
Information on OTC Drug Labels
In 1999, the FDA created a regulation to simplify and standardize the Drug Facts label. It required most OTC drugs to follow the new format and content requirements by May 2002.
These labels are short, simple and typically have six main parts. Some labels include a seventh section with a phone number to call if you have questions or comments.

- Active Ingredient and Purpose: This section lists the ingredient that makes the drug work. It also lists what the active ingredient does and how much of it is in each dose. In this example, the drug contains 500 mg of acetaminophen per caplet, and its purpose is to relieve pain and reduce fever. “It is very important to check [this information] to understand what will be the effect of the drug and to avoid taking other drugs with the same ingredients by mistake,” Hobeika said.
- Uses: This part of the label tells you what symptoms and conditions the drug can treat. It’s important to use the right drug for your symptoms.
- Warnings: This section is typically the longest one on the Drug Facts label. It tells you about any severe side effects or drug interactions that can occur and describes who should not use the drug. It tells you when to stop using the drug and when to consult your doctor and/or pharmacist.
- Directions: This label section tells you when, how and how often to take the drug. Remember that children may have different instructions from adults. “Do not take more than what the label says before asking your doctor,” Hobeika said. “Taking a higher dose than what is recommended on the drug label can be dangerous.”
- Other Information: This part of the label tells you how to safely store the drug. Some drugs may be sensitive to heat or moisture.
- Inactive Ingredients: In addition to the active ingredient that makes the drug work, medications also have ingredients like dyes, preservatives and flavoring agents. “It is important to check this information to help avoid ingredients that may cause an allergic reaction,” Hobeika said.
- Questions or Comments: This section includes contact information consumers can use if they have questions about the product. It may not exist on all Drug Facts labels.
Prescription Drug Labels
Prescription drug labels are more complicated than OTC drug labels. According to Consumer Reports, more than half a million Americans misinterpret prescription drug labels each year.
The FDA doesn’t regulate pharmacy labels. Warning information printed or placed on the bottle as stickers may vary by pharmacy.
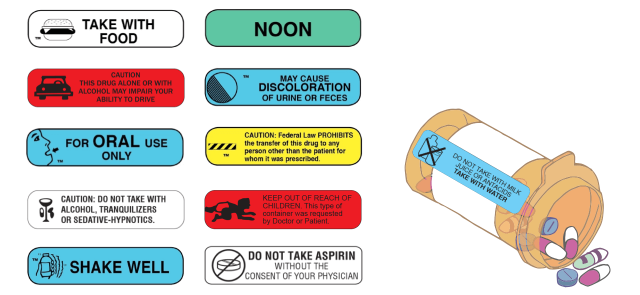
For example, Consumer Reports staffers filled prescriptions for Warfarin at different pharmacies. They found that packaging from Target, Walgreens, CVS, Costco and Walmart included different numbers of warnings for the same prescription. Walmart didn’t include any warnings on the first attempt to fill the prescription.
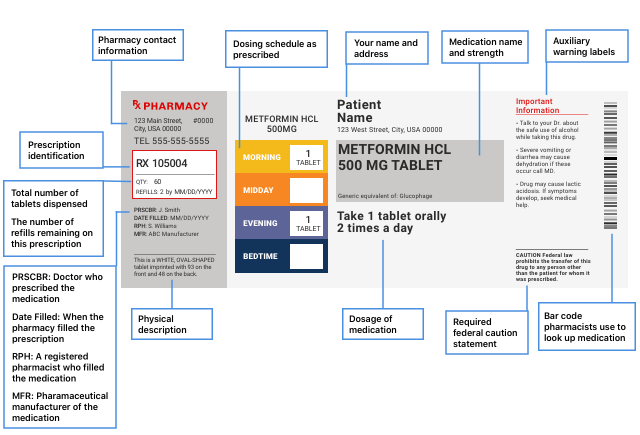
Pharmacy Information Sheets
The drug label printed on prescription drug packaging doesn’t tell you all the safety information for the drug, so pharmacies will give you a pharmacy information sheet. On this sheet, look for warnings or cautions, drug uses, how to use the drug, side effects, precautions, drug interactions, overdose information and how to store the drug.

However, Dr. Michael Carome, director of Public Citizen’s Health Research Group, said in an interview with Drugwatch that the pharmacy information sheet might be incomplete.
“Lots of [prescription] drugs have pharmacy information sheets, and often … those information sheets have not been reviewed by the FDA,” Carome said. “And the information may not be as accurate in terms of things to watch out for.”
When Consumer Reports filled prescriptions for Warfarin, it found “incomplete or hard-to-read package inserts — and in four of five cases, a dangerous omission that violated an FDA regulation.”
Carome recommends finding the official FDA-approved prescribing information on the drug manufacturer’s website or on DailyMed, a site administered by the National Institutes of Health.
Prescribing Information
According to the FDA, the approved prescribing information — also called the package insert or professional labeling — is the actual prescription drug label. This information is several pages long and may contain about 17 sections. It’s intended to help providers properly prescribe the medication, and the FDA updates it regularly on DailyMed.
- Is based on human data when possible
- Is accurate and informative
- Is a summary for the effective and safe use of the drug
- Does not make claims or suggest uses if evidence is lacking
- Is not false, misleading or promotional
Prescribing information can be more complicated to read, but it includes more detailed safety information than the pharmacy drug label or information sheet. Plus, the FDA has reviewed it for accuracy, unlike information provided to you by the pharmacy.
If you have trouble understanding prescribing information, your doctor or pharmacist might be able to help clarify anything you find confusing.
In 2006, the FDA issued a final rule that changed the format of product labels to make it easier for health care professionals.
“The label is now divided into highlights of prescribing information, contents of the full prescribing information (FPI) and the FPI,” Hobeika said. “The highlights section is a half-page summary of the information that health care practitioners most commonly refer to and consider as most important.”
Highlights of Prescribing Information

- Name of Drug. The very first section tells you the name of the drug. It shows the brand name and the generic name, or active ingredient, in parentheses. This section also tells you what formulation it is (injection, oral, etc.) and the year the FDA approved the drug.
- Black Box Warning. Not all drugs carry a Black Box warning. These are the most serious warnings the FDA requires. They are added to a label if the drug may cause severe adverse effects or present serious risks. If there is a black box warning for the drug, it will be at the top of the page. This warning provides the most important safety information about the drug. In the case of Coumadin, it warns about the risks of major or fatal bleeding.
- Recent Major Changes. Below the black box warning, you will find any recent major changes to the prescribing information and the date they were made. The FDA will add or remove warnings based on new research that finds evidence that warrants a change. If you have been taking the same medication for a while, it is a good idea to check this section periodically to see if there are new warnings. However, this section is not always included.
- Indications and Usage. This section lists all of the conditions the FDA has approved the drug to treat. Sometimes, health care providers can legally prescribe drugs for off-label uses. These are uses not approved by the FDA, and they might not be supported by data that proves safety or effectiveness. All the information in the prescribing information is specifically for FDA-approved uses only.
- Dosage and Administration. You can find information about the recommended amount of the drug and how to give it to a patient here. For example, the Coumadin dose depends on tests a health care provider runs, so it is specific to the patient.
- Dosage Forms and Strengths. All of the different doses available from the manufacturer are listed here. Providers can recommend any dose and may increase or decrease the dose over time. Coumadin starts at 1 mg and goes up to 10 mg in its pill form, while the drug for injection comes in a vial containing 5 mg of powder. Check the label on your prescription package from the pharmacy for the dose that your provider has prescribed for you.
- Contraindications. Depending on certain symptoms and medical conditions, not all medications are safe for everyone. This section tells providers who should not take the drug.
- Warnings and Precautions. Warnings and precautions tell doctors about serious conditions that can occur in people taking the drug. It informs them about health problems to watch for in their patients who take the drug. For example, people taking Coumadin might suffer rare tissue death or gangrene that may lead to amputation.
- Adverse Reactions. Look here for the most common side effects experienced by people taking the drug. Coumadin’s most common reactions are fatal and nonfatal bleeding. This section also provides contact information to report side effects to the manufacturer and the FDA.
- Drug Interactions. Drugs listed in this section may interact with the medication, increasing or decreasing its effectiveness or causing side effects.
- Use in Specific Populations. The drug may affect people differently depending on characteristics like age, gender, race, pregnancy or the presence of kidney or liver impairment. This section warns about safety or effectiveness concerns in these groups.
Full Prescribing Information
The full prescribing information elaborates on the overview provided in the highlights section. This part of the prescribing information is the most complicated because manufacturers typically write it for health care providers, scientists and researchers —, not patients.

In addition to the highlights, the full prescribing information contains details on how the drug works, how long it lasts in the body and how the body gets rid of the drug. It even talks about animal studies, which might provide information about whether or not the drug causes cancer or infertility.
The manufacturers also share results of clinical trials, data showing how well the drug works and the side effects seen in those clinical trials.
In an interview with Drugwatch, Diana Zuckerman, president of the National Center for Health Research, recommended patients consider the following questions and tips when reading clinical trial data on drug labels:
- Were patients in the study like you in terms of age, sex, race and other characteristics? Look for at least 30 patients similar to you in the study. Otherwise, it might not provide you with the most relevant data if people in the study are different from you.
- Were patients in the study like you in terms of medical diagnosis? For example, if it’s a cancer drug, did the study involve people with your type of cancer? The same goes for drugs that treat other diseases. Data might be less relevant to you if you are not represented by patients in the study.
- Is the drug tested in people who failed at other treatments? The FDA approves some higher-risk drugs for people with no other treatment options.

Medication Guides
If a drug has an FDA-approved medication guide, pharmacists are supposed to give it to patients with their prescription. Unfortunately, this doesn’t always happen. Plus, not all drugs have these guides.
The FDA requires manufacturers to write medication guides in easy-to-understand language for patients. You can find these medication guides on the drug manufacturer’s website or on DailyMed.
As an example, the blood thinner Coumadin, also known under the generic name Warfarin, has a medication guide. Similar to the Drug Facts on an OTC label, the medication guide tells patients the most important prescription drug information in simple language. For example, this is the medication guide for the blood thinner Coumadin, also known under the generic name Warfarin.
Information in a Medication Guide

- What is the most important information I should know about the drug? The most serious risks, side effects and symptoms you should look for are detailed here
- What is the drug? This section lists the condition or symptoms the drug treats.
- Who should not take the drug? This part describes diseases or health conditions that may worsen with use of the drug. It also addresses people who might have an allergic reaction.
- Before taking the drug. If you have diseases or health conditions listed in this section, talk to your doctor before taking the medication.
- How should I take the drug? This section provides instructions on how to take the drug and reminds people to take the drug only as directed by their doctor.
- What are the possible side effects of the drug? This part includes information on side effects and brief explanations about each possible side effect. It may also include information on symptoms to watch for or tests your provider may run.
- How should I store the drug? Information on how to store the drug is included here. Some drugs are sensitive to heat or cold, while some may need refrigeration.
- What are the ingredients in the drug? Check this section for the drug’s active and inactive ingredients. Tell your provider if you are allergic to any of them.
In 2024, the FDA announced it is proposing new “Patient Medication Information” to replace existing drug labels for outpatient prescriptions, including blood products. This would be a simple, one-page document with clear information to help patients safely use medications. You’ll be able to choose paper or electronic versions.
Elements of FDA’s Proposed New Medication Guide
- Clear, concise information
- Essential information highlighted
- FDA-approved
- Single-page format
- Paper or electronic option
Is Side Effect Information Always Accurate?
Side effect information on drug labels usually comes from clinical trial data collected before a manufacturer sells the drug. Sometimes, the label will get an update based on side effect reports the FDA receives after the drug has been on the market for a while. This information is called postmarketing data.
“If side effects are known, they should always be included on the label, but some side effects aren’t known until years after a product goes on the market,” Zuckerman said.
“If side effects are known, they should always be included on the label, but some side effects aren’t known until years after a product goes on the market.”
Drug manufacturers may face lawsuits if they know about a side effect but fail to warn consumers and doctors.

What Should You Do If You Don’t Understand a Label?
If you have tried reading a drug label and don’t understand it, consider asking for help. Your health care providers, including doctors and pharmacists, are there to assist you.
“Before leaving the doctors’ office, patients should make sure they are well informed about the name of the drug and why they are taking it, the medical conditions this drug treats, how many times per day should they take it, how long will it take this drug to work, when should they stop taking it, are there any side effects that they should know about and any situations where they should not be taking the drug,” Hobeika said.
If you leave your health care provider’s office without the information you need to take your medicine safely and effectively, the pharmacy where you pick up your medication can help. Your pharmacist can answer questions about OTC and prescription medications.
- Check the prescription label to make sure your name is on it. If it isn’t, talk to the pharmacist.
- Check the label to make sure you can read and understand the medicine’s name, directions and colored warning stickers on the packaging. If the letters are too small to read, ask your pharmacist to print them in a larger type.
- Are the directions on the package the same as what you and your provider discussed? If not, tell the pharmacist.
- Ask if there are special instructions on storing the medicine. Should it be kept in the refrigerator or a dry place?
- Ask if there is anything you shouldn’t eat or drink while taking the medicine.
- Ask if it’s safe to take the drug with other prescriptions or OTC medicines you’re taking.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

