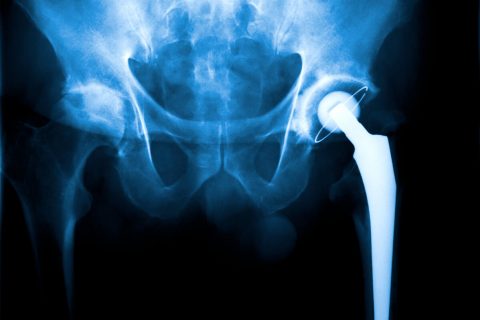
Stryker Hip Replacement Recalls
Stryker hip implant recalls occurred after reports revealed fretting, corrosion and other complications in patients implanted with the devices. The hip manufacturer’s biggest recalls involved Rejuvenate and ABG II Modular stems and LFIT V40 femoral heads. As a result of Stryker’s hip recalls, thousands have filed lawsuits against the manufacturer.
From 2009 to 2013, the Stryker Corporation issued permanent recalls for two of the company’s hip replacement systems and multiple recalls for one of its hip stems.
It recalled the Rejuvenate and ABG II hip implants in 2012, halting all global sales and production of the components. The recall came three months after Stryker issued an urgent field safety notice to surgeons and hospital risk managers describing the potential health hazards associated with the two products.
Complications included corrosion and fretting (wearing away) at modular junctions, which allow metallic ions and particles to leach into a patient’s tissues, bones and bloodstream, causing inflammation, necrosis and systemic metal toxicity (metallosis). The side effects can cause a condition known as metallosis, a type of metal poisoning.
Another hip stem, the Accolade TMZF, has also been associated with adverse health events. The company recalled the product in 2009, 2011, 2012 and 2013 because of packaging and manufacturing errors. About 1,700 units were affected by the recalls, according to the U.S. Food and Drug Administration.
The Accolade TMZF is made of the same proprietary titanium alloy that the Rejuvenate and ABG II are made from, so it may corrode and fret when used with cobalt chromium femoral heads. However, Stryker sells it with ceramic heads today.
Stryker also recalled hip implants in 2008 because of manufacturing issues. After receiving an FDA warning letter, the company stated that its Trident PSL and Hemispherical Acetabular Cups were safe and reliable, but it voluntarily recalled the products because they did not meet company standards in residual tests.
A 2022 study reported that, following a hip replacement, 46.7% of participants experienced a clinically significant improvement, while 15.5% experienced worsened outcomes due to complications such as metallosis, osteolysis or mechanical failure..
Why Stryker Recalled the Rejuvenate and ABG II
In a safety notice issued before the recall, Stryker warned health providers that post-market data revealed that the Rejuvenate and ABG II had a high rate of adverse local tissue reaction. ALTR refers to complications caused by inflammation in the tissue near the implant.
“Following this action, we will work with the medical community to better understand this matter as we continue to evaluate the data.”
Stryker’s official recall announcement, dated July 6, 2012, stated that the company decided to recall the hip replacements when it received the alarming post-market surveillance data.
The modular neck components of the Rejuvenate and ABG II are made of chromium and cobalt, and the stems are coated with titanium. Micromotions at the neck-stem junction generate metallic debris, leading to systemic complications such as metallosis, osteolysis and adverse local tissue reactions.
- Fretting and corrosion in and around the modular neck junction can release excessive metal debris into the surrounding tissue.
- Metal ions in surrounding tissues can result in inflammation leading to an immunological response including metallosis (metal poisoning), necrosis (tissue and bone death) and pain requiring revision surgery.
- Patients with metal sensitivity may have a severe allergic reaction that requires revision surgery.
- Excessive metal debris in the joint space can lead to osteolysis, also known as bone loss, and may require revision surgery.
The U.S. Food and Drug Administration received hundreds of adverse event reports involving the implants in 2012 documenting issues such as severe pain, metallosis and tissue damage. At the time, experts urged anyone who had received the implants to consider having their blood tested for cobalt and chromium levels, even if they hadn’t experienced side effects.
Recall Processing With Broadspire Care Management
Stryker hired Broadspire Care Management to gather information and process claims related to the Rejuvenate and ABG II recall. On Stryker’s website, the company said it would reimburse patients for out-of-pocket medical costs associated with the implants, including revision surgeries performed to remove the implant.
Broadspire is a third-party risk-management company that specializes in handling workers’ compensation, liability and medical case management for large companies. Its role in a previous hip implant recall raised potential red flags about how implant manufacturers appeared to be dealing with injured patients.
A year before the Stryker recall, DePuy, another hip implant manufacturer in the midst of a recall, also turned to Broadspire. The company coordinated patient claims for out-of-pocket medical expenses for people who’d received defective DePuy hip implants.
Normally, the implant manufacturer pays the patients claims and relies on the patient’s own doctor to determine if an implant should be replaced. But under the Broadspire arrangement, the insurance company’s physicians made the determination of whether an implant should be replaced or not.
Broadspire and DePuy sent paperwork to individual doctors, along with promises of $50 payments for each completed packet, to provide the companies with medical release forms for hip implant patients.
Critics claimed Broadspire could use its role to gather medical records and other information on patients, which could then be used against them in court if they filed lawsuits over their faulty implants.
The privacy concerns were raised again when Stryker turned to Broadspire to handle the Rejuvenate and ABG II Modular-Neck Stem recalls. There were again concerns that patients might inadvertently surrender federally protected legal rights.
In the end, thousands of people who experienced complications associated with the ABG II and Rejuvenate hip implants filed lawsuits against Stryker, and the company agreed to settle most of them in 2014.
Stryker LFIT COCR V40 Femoral Head Recalls
The FDA issued a Class II recall of the LFIT Anatomic COCR V40 Femoral Heads on Aug. 29, 2016. The cause of the malfunction was still under investigation when the public notice was posted on Nov. 9, 2016, according to the FDA.
The 2016 recall affected 42,500 devices.
Stryker expanded the list in May 2018. It added eight additional sizes. The company said the devices showed “higher than expected” complication rates. The company did not say how many devices its product safety notice affected this time. But the list of lot numbers ran for 50 pages in the notice.
Health Canada Recalls LFIT V4 COCR Femoral Heads

Health Canada, a Canadian regulatory agency, issued a recall on two separate occasions for the LFIT V4 COCR Femoral Head. The first recall was issued on Sept. 10, 2015 because surgeons were unable to connect the femoral head with the V40 stem during surgery.
The agency issued a second recall on Aug. 24, 2016 because Stryker received large numbers of complaints of taper lock failure in components manufactured before 2011. The taper lock connects the femoral head to the neck, and a failure can lead to inflammation, dislocation and metallosis leading to tissue necrosis and revision surgeries.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




