Wright Medical: Conserve, Dynasty, Profemur & Other Hip Implants
Wright Medical used to sell a variety of hip replacement products including metal-on-metal implants such as Conserve, Dynasty and Profemur implants. But several all-metal models caused complications. Those the implants harmed filed lawsuits against Wright, and the company settled hundreds of them out of court.
Does Wright Medical Still Make Hip Implants?
Once an industry leader in hip and knee replacements, Wright Medical sold off that part of its business in 2013. It now focuses on implants for arms, hands, feet and ankles.
MicroPort Medical purchased Wright’s OrthoRecon hip and knee manufacturing division for $290 million in 2013. The sale came as Wright faced increasing reports of problems with its metal-on-metal hip implants such as its Conserve, Dynasty and Profemur product lines.
People had filed lawsuits against Wright claiming injuries from their hip implants. The company settled hundreds of them out of court. While Wright Medical no longer makes hip replacements, it was legally responsible for faulty hips made while it owned OrthoRecon and for some models MicroPort continued to make for a time after the sale.
Wright Medical Metal-on-Metal Hip Blamed for Complications
The company’s metal-on-metal hips were associated with complications and failure. Consumers alleged that the defective design of MoM devices from Wright’s Conserve, Dynasty and Lineage product lines led to early device failure. These implants showed early failure due to the wear of the metal components, compromising both the biocompatibility and longevity of the device.
MoM hip implants were particularly problematic and are no longer available in the U.S. Friction between metal parts can release microscopic amounts of metal, which can cause metallosis – a serious form of metal poisoning.
- Early device failure
- Metallosis – metal poisoning or metal toxicity
- Infection
- Bone and tissue damage
The company’s total hip replacements included its ceramic hip system, hard-bearing surfaces and proprietary neck modularity. Wright’s use of alumina oxide ceramic in its Lineage liners and femoral heads were considered industry-leading ceramic-on-ceramic hip system.
Study: Wright Conserve Hips High Failure Rate
A 2016 study found high sooner-than-expected failure rates among 108 Wright Conserve MoM hips attributed to poor wear resistance and the resultant accelerated surface degradation of the metal components. Researchers found roughly two in 10 Conserve hips failed over the scope of their analysis – November 2005 and December 2010. Early failure often necessitated revision surgery due to loosening or catastrophic failure (fracture of the acetabular cup or femoral head). Usually, only one in 10 hip implants will fail within 15 years of being implanted.
The hips were implanted in 98 patients – some patients having both hips replaced. On average, patients receiving Conserve hips required revision surgery after only four years and three months following the original surgery to implant them.
Problems with Wright Conserve Hip Replacements

Conserve hip implants began demonstrating higher than normal failure rates. The U.S. Food and Drug Administration received reports of more than 200 adverse events that caused severe pain in the hip and groin, soft tissue necrosis, inflammatory response and significant bone loss leading to implant loosening, early failure and metal toxicity in the blood.
The Conserve hip replacement line was created to meet the demand for durable implants for younger people with active lifestyles. According to Wright, Conserve implants provided excellent range of motion, low particle wear and long-term dependability with reduced chances of soft tissue impingement, which can lead to dislocation.
Problems with Wright Profemur Z Hips Implants
Shortly after Wright sold its hip and knee manufacturing unit to MicroPort, the new manufacturer started receiving reports of failures with the Profemur modular neck. In 2015, MicroPort voluntarily recalled the Wright Medical-designed Profemur Neck Varus/Valgus CoCr 8 Degree modular neck due to the risk of fractures.
- Corrosion
- Fretting – wear damage from two surfaces rubbing together
- Femoral neck fracture
- Fractures
The FDA classified MicroPort’s action as a Class I recall: “a situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.” Though made on MicroPort’s watch, Wright Medical assumed legal liability for the devices.
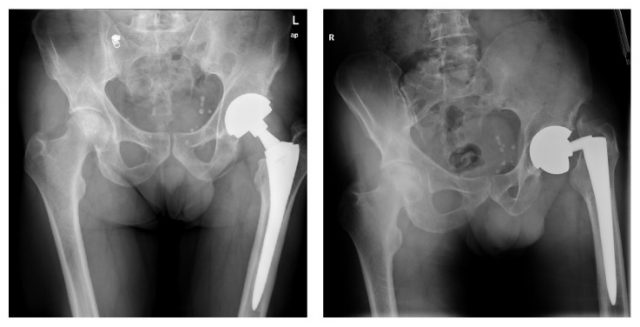
The Conserve Total Hip acetabular cup and ball were designed to be used with a number of stems, including the Profemur Z. The Profemur Z had a modular neck and stem system that was made of titanium alloy. Modular necks and stems allow surgeons to customize length for each patient to provide a better fit.
Wright Medical Lineage and Dynasty Hip Replacements
Wright Medical also manufactured two more model lines of hip replacements, the Lineage and the Dynasty, which were made of different material combinations.
- Ceramic-on-ceramic
- Metal-on-polyethylene (plastic)
- Ceramic-on-metal
- Metal-on-metal
These hip implants also came in a variety of sizes.
Wright Hips: Metallosis Symptoms and Complications
Wright hip replacements were one of several lines of MoM hips associated with metallosis, which can affect the skin, nervous system and internal organs, causing potential damage to vital structures such as muscles, ligaments and nerves. People may not experience any symptoms, but doctors can detect it through blood tests that can identify elevated levels of metal ions.
- Clicking or other sounds coming from implant
- Infection
- Implant loosening
- Skin rashes
If left untreated, metallosis can result in several serious complications.
- Auditory impairment that can result in deafness
- Cognitive impairment or memory issues
- Depression, anxiety or other mental health issues
- Heart problems, including heart failure
- Nerve problems
- Thyroid problems
- Visual impairment that may result in blindness
Treating metallosis typically requires removing and replacing the implant.
Wright Settles Lawsuits Over Faulty Hip Replacements
Wright faced approximately 2,000 hip replacement lawsuits and agreed to two settlements to resolve all of them. In 2017, the Tennessee-based device maker offered to settle hundreds of personal injury lawsuits.
People across the U.S. alleged that their Wright implants caused medical complications. Many of them required revision surgeries to remove the device and treat their injuries.
- Conserve
- Dynasty
- Lineage
- Profemur
Wright paid $240 million to settle 1,292 claims against its Dynasty and Lineage implants in November 2016. The agreement didn’t cover hundreds of cases, however.
The company announced in October 2017 it would settle the remaining 600 or so lawsuits still active for an additional $90 million. Following the settlement, the court closed the Wright MDL in June 2018. Wright expected to deliver the final settlement payments in September 2019.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

