Humira Side Effects & Warnings
Humira is part of a class of biologics known as TNF blockers. These medications work by suppressing the immune system. While these drugs may be effective in treating symptoms of various inflammatory diseases, they also put users at greater risk of serious and even deadly infections and cancers.
A black box warning in Humira’s label highlights the risk of serious infections leading to hospitalization or death, including TB, bacterial sepsis, invasive fungal infections and infections due to opportunistic pathogens. It also features cancers, notably lymphoma and hepatosplenic T-cell lymphoma.
Other warnings listed in Humira’s label include severe allergic reactions, hepatitis b reactivation, neurological reactions, blood reactions, worsening congestive heart failure and lupus-like syndrome.
Common Side Effects
One of the most common side effects of Humira is a reaction at the injection site. The U.S. Food and Drug Administration received reports of more than 10,000 cases of injection-site reactions related to the medication in 2014. Injection-site reactions include bleeding, redness, rash, swelling, pain, itching or bruising. Symptoms typically go away within a few days. However, patients should call a doctor immediately if they have pain, redness or swelling around the injection site that doesn’t go away within a few days or gets worse.
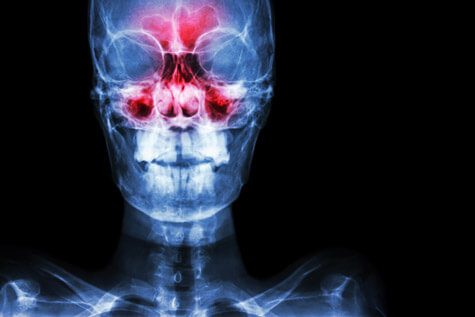
During clinical trials involving nearly 1,400 people, 17% of patients treated with Humira reported upper respiratory infection compared with 13% of people who received a placebo. Another 11% of Humira-treated patients reported sinusitis while only 9% of people given the placebo reported the condition.
Headache and rash were each reported by 12% of Humira-treated patients. Eight percent and 6% of placebo-treated patients reported headache and rash, respectively.
| Side Effects | Humira (705 patients) | Placebo (690 patients) |
|---|---|---|
| Upper Respiratory Infection | 17% | 13% |
| Sinusitis | 11% | 9% |
| Headache | 12% | 8% |
| Rash | 12% | 6% |
Other reported side effects were flu-like symptoms, nausea, abdominal pain, abnormal laboratory tests, high amounts of cholesterol in the blood, high levels of fat particles in the blood, blood in urine, increased alkaline phosphatase, accidental injury, back pain, urinary tract infection and hypertension.
Serious Infections
Serious and even fatal infections are a recognized risk of Humira. These serious infections may be caused by viruses, fungi or bacteria. They can spread throughout the body and may lead to hospitalization and death.
The risk of serious infection may be even greater in patients who use Humira at the same time as other biologics, including Orencia (abatacept), Kineret (anakinra), Remicade (infliximab), Enbrel (etanercept), Cimzia (certolizumab pegol) or Simponi (golimumab). Some patients who were treated with rituximab (Rituxan) followed by Tumor Necrosis Factor-alpha (TNF blockers) also showed a higher rate of serious infections.
In addition to a tuberculosis (TB) warning, today’s Humira label carries a boxed warning for invasive fungal infections, such as histoplasmosis, as well as Legionella and Listeria infections. But that was not always the case. For years, Humira’s black boxed warning only included the risk of TB.
Tuberculosis (TB)
Humira’s black box warning clearly advises patients be tested for TB before starting Humira. Patients who test positive for TB should be treated before taking the drug. Doctors should monitor all patients closely for signs and symptoms of TB during treatment with Humira, even if the initial latent TB test is negative.
“Cases of reactivation of tuberculosis and new onset tuberculosis infections have been reported in patients receiving Humira, including patients who have previously received treatment for latent or active tuberculosis,” according to the FDA.
Symptoms related to TB include a cough, fever, weight loss or loss of body fat and muscle. People considering Humira should let the prescribing doctor know if they have TB or have been in close contact with someone with TB. Also let the doctor know if the patient was born in, lives in or traveled to a place where there is more risk for getting TB.
According to the lawsuit, the man was given a shot of Humira and shortly thereafter began feeling numb from the waist down. He was then diagnosed with TB, according to the suit.
Invasive Fungal Infections
In September 2008, the FDA announced it would require makers of TNF blockers, like Humira, to highlight information about the risk of invasive fungal infections in a black box warning as well as in the warnings section of the drug’s prescribing information.
The agency notified health care professionals that it had received reports of people developing pulmonary and disseminated histoplasmosis, coccidioidomycosis, blastomycosis and other infections while taking TNF blockers, such as Humira, and doctors were not consistently identifying the infections.
“In some patients, the diagnosis of histoplasmosis was initially unrecognized and antifungal treatment was delayed. Some of these patients died from histoplasmosis. There were also deaths in patients with coccidioidomycosis and blastomycosis.”
Legionella and Listeria Bacteria
In September 2011, the FDA announced it had added information about infection with Legionella and Listeria bacteria to the black box warning for all TNF blockers, including Humira.
The agency explained it had identified cases of Legionella pneumonia in people treated with TNF blockers. The FDA searched its Adverse Event Reporting System and identified reports of 80 patients who developed Legionella pneumonia between 1999 and 2010 after taking Humira or other TNF blockers. At least 14 people died.
In addition, the FDA searched English-language medical literature and identified case reports of 23 patients who developed Legionella pneumonia after being treated with TNF blockers. Four patients experienced severe pneumonia requiring mechanical ventilation; five patients received treatment in a hospital intensive-care unit and three patients died.
The FDA has also received reports of people taking TNF blockers and developing serious infections due to Listeria monocytogenes. The agency searched English-language medical literature and identified 26 published cases of Listeria infection in patients treated with TNF blockers. Infections included meningitis, bacteremia, endophthalmitis and sepsis. There were seven reported fatalities.
“In addition, FDA identified fatal Listeria infections in a review of data regarding laboratory-confirmed infections that occurred in pre-marketing phase 2 and phase 3 clinical trials and from post-marketing surveillance,” the agency said.
Cancer
Patients taking TNF blockers have developed lymphoma, skin cancer (including basal cell and squamous cell) and leukemia. Some children, teenagers and young adults have developed hepatosplenic T-cell lymphoma, a rare, often fatal cancer of white blood cells.
Lymphomas, Leukemia and Melanoma
In June 2008, the FDA announced it was investigating 30 reports of cancer in children and young adults who were treated with TNF blockers for Juvenile Idiopathic Arthritis, Crohn’s disease or other diseases.
Reports of cancer were submitted to the FDA’s Adverse Event Reporting System over 10 years, beginning in 1998 — after the first TNF blocker hit the market — through April 29, 2008. The cases involved children and young adults who took TNF blockers with methotrexate, azathioprine or 6-mercaptopurine.
About half the cancers were lymphomas, including both Hodgkin and non-Hodgkin lymphoma; other cancers reported included leukemia, melanoma and solid organ cancers.
In a follow-up announcement in August 2009, the FDA said it had completed its analysis and concluded that there is an increased risk of lymphoma and other cancers associated with the use of TNF blockers in children and adolescents. The agency added the new safety information to the boxed warning for Humira and the other TNF blockers.
In addition, the FDA added new warnings about leukemia and new-onset psoriasis in patients treated with TNF blockers.
Hepatosplenic T-cell Lymphoma
The FDA put out two alerts in 2011 regarding TNF blockers and the development of cancer. First, in April, the FDA said it continued to receive reports of hepatosplenic T-cell lymphoma (HSTCL).
Reports were primarily in adolescents and young adults being treated for Crohn’s disease and ulcerative colitis with TNF blockers as well as azathioprine and/or mercaptopurine. One patient was being treated for psoriasis and two patients were being treated for rheumatoid arthritis.
The FDA reviewed data from the Adverse Event Reporting System database, literature and the HSTCL Cancer Survivors’ Network and identified two cases of HSTCL with Humira and five cases with the combination of Humira and Remicade.
Cases were reported between the time TNF blocker marketing started and December 31, 2010. Of the five cases of Remicade or Humira use, four involved concomitant use of mercaptopurine or azathioprine.
FDA Announces ‘Enhanced Safety Surveillance’ for Humira
In a second 2011 safety communication, the FDA announced that it is requiring manufacturers of TNF blockers to “perform enhanced safety surveillance” for TNF blockers.

As part of the enhanced safety surveillance, manufacturers must conduct in-depth follow-up of reports of cancer cases and submit all reports of cancer in pediatric and young adult patients to the FDA within 15 days of becoming aware of the report. The manufacturers must also provide annual summaries and assessments.
“This type of safety surveillance is important for our improved understanding of malignancies in pediatric and young adult patients treated with TNF blockers because it will allow [the] FDA to more completely capture and analyze all reported malignancies based on more complete and consistent reports,” the FDA said.
The agency says it will periodically re-evaluate the enhanced surveillance requirement through 2021.
In early 2022, the FDA designated Cyltezo (adalimumab-adbm) as an interchangeable biosimilar to Humira. Since then, pharmacies have been allowed to substitute Cyltezo for Humira without specification in the prescription.
Severe Allergic Reactions
People have experienced anaphylaxis (severe, life-threatening allergic reaction) and angioedema (swelling) following Humira administration.
In clinical trials of Humira in adults, patients suffered allergic reactions, including allergic rash, anaphylaxis and urticaria (skin rash).
In a study of Humira in children with Juvenile Idiopathic Arthritis, 6% of patients suffered allergic reactions — including allergic rash — within the first 48 weeks of treatment. About 5% of patients in a study of Humira in children with Crohn’s disease suffered allergic reactions.
Signs of a serious allergic reaction include hives, trouble breathing, and swelling of the face, eyes, lips or mouth. Patients should call their doctor or get medical help immediately if they experience symptoms of an allergic reaction.
Hepatitis B Reactivation
People who carry hepatitis B virus (HBV) in their blood should be extra cautious when deciding whether to use Humira because the virus can become active during treatment.
“In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal,” according to Humira’s label. “The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation.”
Patients should undergo blood tests before, during and for several months after treatment with Humira.
- Muscle aches
- Clay-colored bowel movements
- Feeling very tired
- Fever
- Dark urine
- Chills
- Skin or eyes look yellow
- Stomach discomfort
- Little or no appetite
- Skin rash
- Vomiting
Neurological Reactions
Humira’s label warns consumers that the medicine has been associated with central and peripheral nervous system demyelinating disease, although causality has not been proven. This includes multiple sclerosis (MS), optic neuritis and Guillain-Barré syndrome.
Some people experienced symptoms of these disorders for the first time following Humira use; others suffered from worsening of symptoms they already had.
“Exercise caution in considering the use of HUMIRA in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders,” the Humira label states.
Blood Reactions
Though rare, some people have experienced blood reactions with use of TNF blockers, including Humira.
Humira’s label specifically warns of reports of pancytopenia, a condition in which the body has too few red blood cells, white blood cells and platelets.
- Aplastic Anemia
- Not enough new blood cells
- Thrombocytopenia
- Not enough platelets in the blood
- Leukopenia
- Not enough white blood cells
Signs and symptoms that may indicate blood dyscrasias or infection include persistent fever, bruising, bleeding and pallor. Anyone who experiences these symptoms while using Humira should contact a doctor.
Heart Failure
Humira may cause new heart failure or worsening of heart failure patients already have.
The drug’s label warns of cases of worsening congestive heart failure (CHF) with Humira, though the medicine has not been formally studied in patients with the condition.
Other Humira Warnings
Humira may cause immune reactions, including a lupus-like syndrome. Symptoms may improve when patients stop taking Humira, though they should not stop treatment without consulting a doctor. Symptoms include chest discomfort or pain that does not go away, shortness of breath, joint pain or a rash on the cheeks or arms that gets worse in the sun.
In addition, liver problems — including ones that can lead to liver failure and death — can happen in people who use TNF blockers, such as Humira. Symptoms of liver problems include fatigue, yellow-looking skin or eyes, poor appetite or vomiting, and pain on the right side of your stomach.
Some Humira users suffered new psoriasis or worsening of psoriasis they already had. Signs of psoriasis include red scaly patches or raised pus-filled bumps.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

