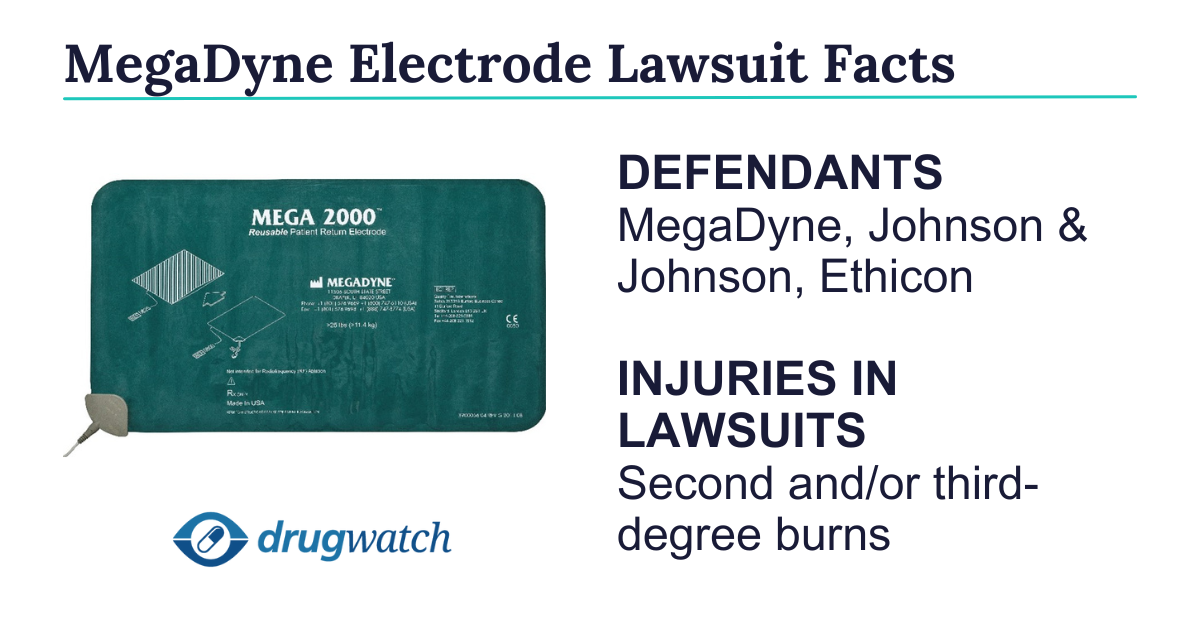
MegaDyne Electrode Lawsuit
MegaDyne electrode lawsuits claim that the devices used for surgery are defective and cause serious burn injuries. Lawyers claim MegaDyne’s parent company, Johnson & Johnson, didn’t properly warn about the risk. MegaDyne issued recalls for more than 21,000 devices.
Article Continues Below
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- MDL
- None yet
- Settlements/Verdicts
- No verdicts or settlements for injury cases yet
Latest MegaDyne Electrode Lawsuit Updates
As of April 2025, MegaDyne electrode lawsuits are still in the early stages. Lawyers are investigating cases from people who suffered second and/or third-degree burns after undergoing surgery with a MegaDyne Patient Return Electrode.
We do the legal research by talking to lawyers and reviewing court documents to bring you the latest updates on MegaDyne Electrode lawsuits.
Timeline of MegaDyne Electrode Lawsuits
-
August 2024:
Lawyers are investigating claims of serious burns with Mega 2000 and Mega Soft Patient Return Electrodes. We don’t have much else to report yet, since this litigation is in the early stages.
-
May 2024:
MegaDyne sent a recall letter for Mega Soft Pediatric Patient Return Electrodes devices with instructions on how to return the device. The company also announced it would discontinue the 0840 pediatric pad product.
-
December 2023:
MegaDyne sent an Urgent Medical Device Correction letter specifically for the Mega Soft Electrodes to warn customers not to use the product in children under the age of 12 because of burn reports.
-
June 2023:
MegaDyne sent an Urgent Medical Device Correction letter to customers for all users of Mega 2000 and Mega Soft Reusable Patient Return Electrodes after it received reports of patient burns.
MegaDyne Electrode Devices Included in Lawsuits
Several models of MegaDyne Mega 2000 and Mega Soft Reusable Patient Return Electrode devices are included in lawsuits. These devices are soft pads that contain an electric current and are used during electrosurgery. Mega 2000 and Mega Soft are used on adults and children.
- Mega 2000 Patient Return Electrode 0800
- Mega Soft Reusable Patient Return Electrode 0830
- Mega Soft Dual Reusable Patient Return Electrode 0835
- Mega Soft Pediatric Patient Return Electrode 0840
- Mega Soft Universal Patient Return Electrode 0845
- Mega Soft Universal Dual Patient Return Electrode 0846
- Mega Soft Universal Plus Patient Return Electrode 0847
- Mega Soft Universal Plus Dual Patient Return Electrode 0848
In this type of surgery, a pen-like device uses an electric current to heat or cut tissue or to stop bleeding. The current comes from an electrosurgical generator. The MegaDyne electrode pad is placed under a patient’s back as they lie on the surgical table.
The Reusable Patient Return Electrode pad conducts the electric current through the patient’s tissue back to the electrosurgical unit. This prevents overheating.
Do I Qualify for a MegaDyne Electrode Lawsuit?
If you or your child had surgery using a MegaDyne Mega 2000 and Mega Soft Reusable Patient Return Electrode and suffered second- or third-degree burns, you may qualify for a lawsuit.
According to the FDA, “These burns may be as serious as third-degree burns requiring medical intervention and may lead to a longer hospital stay, scarring, and potentially more surgeries for pediatric and adult patients.”
“These burns may be as serious as third-degree burns requiring medical intervention and may lead to a longer hospital stay, scarring, and potentially more surgeries for pediatric and adult patients.”
The patient must have had electrosurgery using the MegaDyne product between March 11, 2021 and July 31, 2024.
These are just guidelines, and you should contact a MegaDyne Reusable Patient Return Electrode lawyer for a free case evaluation to find out about your specific case.
Keep in mind that each state has a time limit to file a claim called the statute of limitations. Depending on the state you live in, you may not qualify based on state laws. Make sure you contact an attorney right away to preserve your legal right to file a claim.
MegaDyne Electrode Recall
MegaDyne issued a recall for all Ethicon MegaDyne Mega Soft Pediatric Patient Return Electrodes on May 10, 2024. The company issued the recall because of reports of patient burns. These devices are being removed from the market and discontinued.
According to a company investigation and testing, several factors could cause these thermal injuries. Though the recall notice doesn’t specifically state what those factors are, it said between 2018 and May 2024, there were 4 reported injuries and no deaths.
“The combination of these conditions [that cause thermal injuries] may be more likely when the pad is used with infants and small children.”
“The combination of these conditions may be more likely when the pad is used with infants and small children. Because the pediatric pad is designed for patients between 0.8 to 50 pounds, which would be predominantly patients under the age of 12, the decision was made to discontinue and recall the 0840 pediatric pad product,” MegaDyne said in its recall notice.
Prior to the recall and discontinuation of Mega Soft Pediatric Patient Return Electrodes in May 2024, MegaDyne issued a recall (device correction) in June 2023 for 21,200 Mega 2000 and Mega Soft devices in the U.S. The devices were distributed between March 11, 2023 and May 9, 2023.
- Mega 2000 Patient Return Electrode 0800
- Mega Soft Reusable Patient Return Electrode 0830
- Mega Soft Dual Reusable Patient Return Electrode 0835
- Mega Soft Pediatric Patient Return Electrode 0840
- Mega Soft Universal Patient Return Electrode 0845
- Mega Soft Universal Dual Patient Return Electrode 0846
- Mega Soft Universal Plus Patient Return Electrode 0847
- Mega Soft Universal Plus Dual Patient Return Electrode 0848
As of July 31, 2024, the company has reported 63 injuries and zero deaths related to these devices, according to the FDA. The agency has classified this recall as a Class I, the most serious recall. This means using these devices can cause serious injuries or death.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.



