Lemtrada Side Effects
Lemtrada can cause severe side effects, including stroke, cancer and autoimmune disorders. FDA data shows nearly 70% of reported cases are serious. The FDA restricts the distribution of Lemtrada infusions to patients and health care providers enrolled in a special program designed to protect patients.
Article Continues Below
Board-certified physicians medically review Drugwatch.com content to ensure its accuracy and quality.
Drugwatch.com partners with Physicians’ Review Network Inc. to enlist specialists. PRN is a nationally recognized leader in providing independent medical reviews.
Reviewer specialties include internal medicine, gastroenterology, oncology, orthopedic surgery and psychiatry.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Common Side Effects
- Rash, headache, fever, cold-like symptoms, nausea, infection, fatigue, trouble sleeping
- Black Box Warning
- Autoimmunity, infusion reactions, stroke, cancer
- Distribution Restricted
- Because of the risk of serious side effects, the drug is only available through the Lemtrada Risk Evaluation and Mitigation Strategy (REMS) Program.
- Manufacturer
- Sanofi Genzyme
Latest Side Effects Information for Lemtrada
As of September 2024, headache, fatigue, rash and fever were the most common Lemtrada side effects reported to the U.S. Food and Drug Administration. Each of these symptoms made up 8% or more of all reported side effects.
| FDA Adverse Events Reporting System (FAERS) Data for Lemtrada Side Effects | |
|---|---|
| Total cases reported | 5,749 |
| Serious cases (including deaths) | 4,005 |
| Deaths | 194 |
Disclaimer: Reports sent to the FDA don’t necessarily mean the drug caused an adverse event. Consult a health care professional before stopping or changing medication.
Most reported side effects are mild. However, by FDA standards, nearly 70% of all side effects in the agency’s FAERS Database were severe. More severe common side effects include difficulty breathing, severe weakness, urinary tract infection and a condition called immune thrombocytopenia. This condition prevents blood from clotting, which can lead to excessive blood loss.
Common Side Effects of Lemtrada
The most common Lemtrada side effects match those found in clinical trials. The trial participants reported headache, fatigue, rash and fever as common side effects.
- Back pain
- Diarrhea
- Dizziness
- Fatigue
- Fever
- Fungal infection
- Headache
- Herpes viral infection
- Hives
- Itching
- Joint pain
- Mouth pain
- Nausea
- Pain in arms or legs
- Rash
- Sinus infection
- Sore throat
- Stomach pain
- Sudden redness in the face, neck or chest
- Swelling of nose and throat
- Thyroid problems
- Tingling sensation
- Trouble sleeping
- Upper respiratory infection
- Urinary tract infection
- Vomiting
While Lemtrada does not directly cause infections, it may increase the patient’s risk of contracting one. This is why some patients experience side effects such as sinus or fungal infections, herpes and urinary tract infections.
Serious Lemtrada Side Effects
Lemtrada can cause serious side effects, including issues with the immune system, severe reactions, strokes, blood vessel damage and certain types of cancer.
- Artery tearing
- Blood cancers (lymphoma & lymphoproliferative disorders)
- Infusion reactions
- Severe autoimmune disorders
- Skin cancer (melanoma)
- Stroke
- Thyroid cancer
Due to these risks, Lemtrada is only available through a special program called the Risk Evaluation and Mitigation Strategy (REMS) Program to ensure patient safety. Additionally, many patients had filed Lemtrada lawsuits, claiming it caused them serious injury.
Lemtrada’s Restricted Distribution
Because Lemtrada carries serious risks, it can only be obtained through the Risk Evaluation and Mitigation Strategy (REMS) program. The FDA requires this program to ensure the benefits of Lemtrada outweigh the risks in all cases.
The REMS program educates and follows everyone involved in the treatment process. This includes patients, healthcare providers, pharmacies and facilities.
The idea is to carefully monitor patients for any potential side effects, including autoimmune conditions and a higher risk of thyroid cancer and melanoma.
The REMS program requires regular tests and check-ups for up to 48 months after the last dose. This can help catch dangerous issues early.
Fatal Side Effects of Lemtrada Identified in Study
A study in BMC Research Notes used a European database and found nine instances in which Lemtrada was likely linked to fatal side effects and one instance that was possibly related. Most deaths happened within a month of treatment.
- Intracerebral Hemorrhage: A woman died from a brain bleed five days after treatment.
- Infections: Several patients died from infectious diseases like pneumonia and listeriosis, which causes severe diarrhea and vomiting, within a few weeks.
- Septic Shock: Some patients experienced severe infections leading to multiple organ failure.
- Autoimmune Reactions: Months after treatment, a few patients died from their immune system attacking their bodies. This caused conditions like autoimmune hepatitis and thrombocytopenia (low platelets).
According to the study’s authors, Lemtrada can cause these serious and sometimes fatal side effects in the first month after treatment.
Stroke and Blood Vessel Tears With Lemtrada
Some people who received Lemtrada have had strokes or tears in the arteries in their neck and head within three days of getting Lemtrada. These problems can be hazardous and might even cause death or long-term disability.
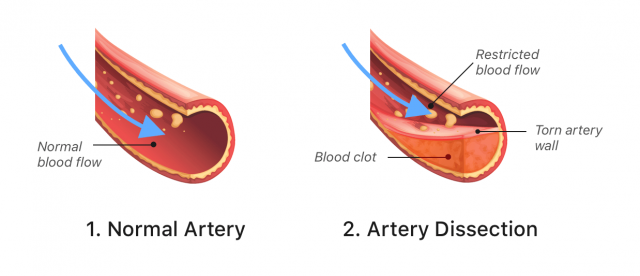
Because of these risks, the FDA added a stroke risk warning to Lemtrada’s label in 2018. In 2020, it added another warning about arterial tearing.
If you or someone you care for is taking Lemtrada, it’s essential to watch for signs of a stroke.
- Sudden numbness or weakness, especially on one side of the body
- Sudden confusion, trouble speaking or understanding speech
- Sudden vision problems in one or both eyes
- Sudden difficulty walking, dizziness or loss of balance
- Sudden severe headache or neck pain
If any of these symptoms happen, seek emergency medical help right away. Most of these serious side effects happen within one day of treatment, but they can occur up to three days later. Doctors will closely monitor patients after giving Lemtrada to keep them safe.
Autoimmunity Side Effects of Lemtrada
Lemtrada can lead to severe autoimmune issues. Autoimmune diseases happen when the immune system mistakenly attacks your body. You may develop conditions where your immune cells attack other organs or tissues in life-threatening ways.
Lemtrada can cause rare autoimmune disorders that can cause blood, bleeding, kidney, liver and thyroid problems, according to Sanofi.
- Acquired Hemophilia A (AH)
- Unexplained bleeding in patients with no history of clotting disorders.
- Anti-GBM Disease
- A serious condition that, if untreated, can cause severe kidney damage, kidney failure or death. It may require dialysis or a kidney transplant.
- Autoimmune Hepatitis (AIH)
- The immune system attacks the liver, causing inflammation. If untreated, this can lead to cirrhosis, liver cancer or liver failure.
- Hemophagocytic Lymphohistiocytosis (HLH)
- A rare, life-threatening condition where the immune system attacks organs and destroys blood cells.
- Hyperthyroidism
- An overactive thyroid.
- Hypothyroidism
- An underactive thyroid.
- Immune Thrombocytopenic Purpura (ITP)
- Low platelet counts leading to severe, life-threatening bleeding.
Researchers writing in the journal Multiple Sclerosis in 2021 discussed the link between Lemtrada and several autoimmune conditions. According to the authors, these risks were so concerning that in 2019, European health authorities limited their use for individuals with existing autoimmune diseases.
Lemtrada Infusion Reactions
Lemtrada (alemtuzumab) might cause infusion reactions for most patients, and around 92% experienced this side effect during clinical trials. However, only 3% of these reactions were considered serious.
Severe reactions are rare, but they can be life-threatening. They may occur during or up to 24 hours after the infusion. To lower the risks, Lemtrada is only given in certified healthcare settings that are prepared to handle emergencies. Healthcare providers carefully monitor you during the infusion and for at least two hours afterward.
- Chest pain
- Fast, slow or irregular heartbeat
- Rash
- Swelling in the mouth
- Swelling in the throat
- Trouble breathing
- Weakness
The doctor typically gives corticosteroids before the first three infusions to decrease the likelihood of a reaction. Doctors may also give antihistamines and fever reducers before, during and after treatment. Even with these precautions, reactions can still occur. In addition, patients will receive antiviral medication to prevent serious infections related to the treatment.
Lemtrada’s Cancer Risk
Lemtrada may increase the risk of some cancers. These include thyroid cancer, skin cancer (melanoma) and blood cancers like lymphoma. While researchers identified these risks in clinical trials, the overall risk is not much higher than in other treatments. However, caution is still necessary.
Thyroid Cancer
Research has associated Lemtrada with an increased risk of thyroid cancer, particularly papillary carcinoma.
- A new lump or swelling in the neck
- A persistent cough
- Changes in voice, such as hoarseness
- Pain in the front of the neck
You should look for these signs if taking Lemtrada. Early detection can significantly improve cancer outcomes.
Skin Cancer (Melanoma)
Patients treated with Lemtrada are at a higher risk of developing melanoma, a severe form of skin cancer. Melanoma often appears as a new or changing mole. It is usually asymmetrical, with irregular borders or color changes.
Regular skin checks are essential for catching melanoma early when it is most treatable.
Lymphoma and Other Blood Cancers
Lemtrada is linked to various lymphoproliferative disorders, including lymphoma and rare conditions like Castleman disease and Burkitt’s lymphoma.
These cancers affect the lymphatic system. Symptoms include swollen lymph nodes, unexplained weight loss, night sweats and fatigue.
Though rare, these conditions are serious and require immediate medical attention.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


