Chantix Recalled Over Potential Cancer Risk
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
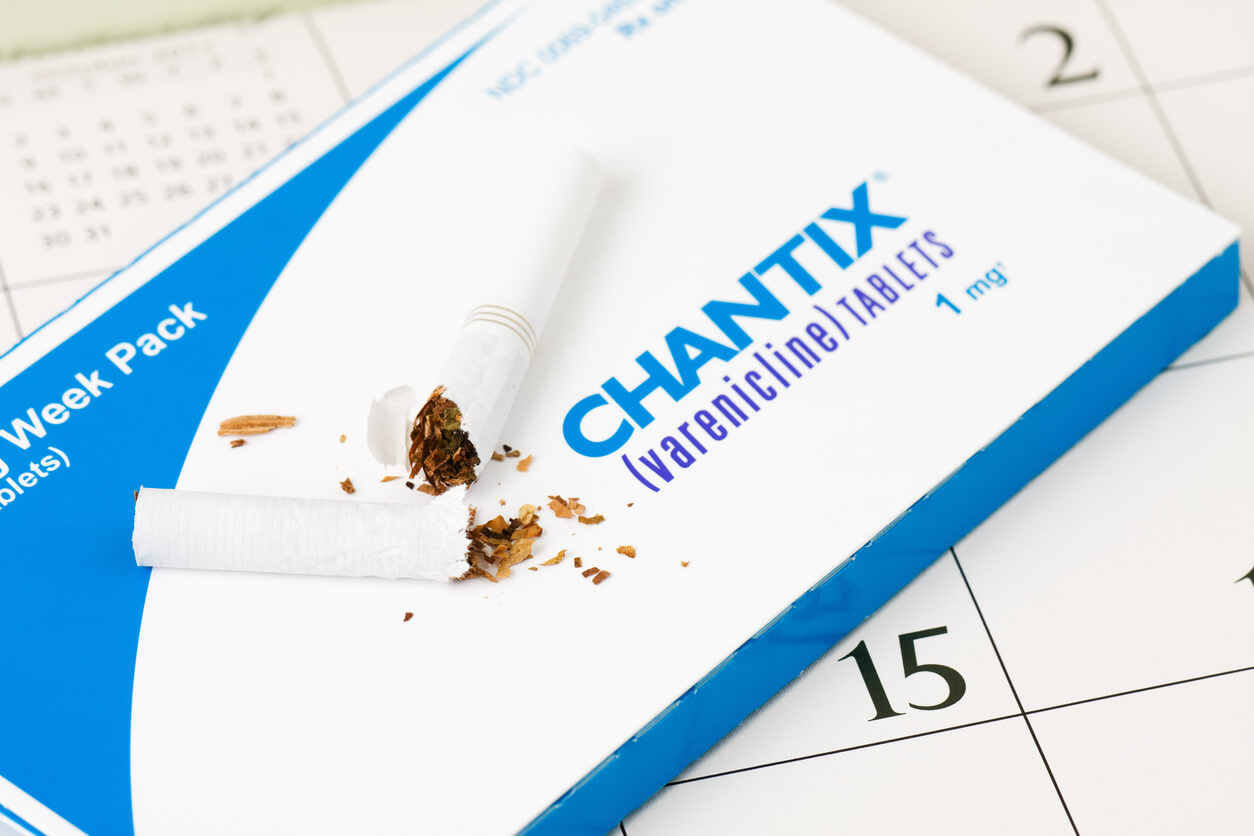
The popular anti-smoking pill Chantix has been recalled by manufacturer Pfizer Inc. due to high levels of an ingredient linked to an increased cancer risk.
The U.S. Food and Drug Administration posted the company’s voluntary recall announcement on Sept. 16 as a public service.
Pfizer said it was recalling all lots of Chantix 0.5 and 1 mg tablets because of N-nitroso-varenicline, known as a nitrosamine, found above the FDA’s acceptable intake limit.
Chantix, the smoking cessation drug, was first approved by the FDA in 2006.
In its announcement, Pfizer said the recall was “precautionary” and there was no immediate risk to patients taking the medication, which is intended for short-term use.
“Chantix has a safety profile that has been established over 15 years of marketing authorization and through a robust clinical program,” the company statement read. “Pfizer believes the benefit/risk profile of Chantix remains positive.”
The day after the recall, the FDA reminded patients to continue taking their current medicine until their doctor prescribes a different treatment, or their pharmacist provides a replacement.
“The health benefits of stopping smoking outweigh the cancer risk from the nitrosamine impurity in varenicline,” the FDA statement read.
According to the FDA, taking nitrosamines at or below acceptable levels does not increase the risk of cancer. The chemical may increase the risk of cancer, according to the FDA, if someone is exposed to high levels over long periods of time.
Lower levels of nitrosamines are common in many foods, including grilled and cured meats, some vegetables and dairy products, and are thought to not cause problems.
However, this latest recall comes as no surprise based on earlier events in 2021.
In June, Pfizer suspended the world-wide distribution of Chantix, while recalling select lots of the product after finding high levels of nitrosamines. Additional wholesale lots within the U.S. were recalled in July, and more in August.
The growing supply disruptions sparked a shortage in recent months, leading to the FDA’s approval of generic products being imported from Canada.
This latest Chantix recall was all-inclusive.
The FDA had requested the earlier testing as part of its investigation into the presence of nitrosamines in various medicines.
In 2020, the FDA asked three companies to recall metformin, a common diabetes drug, after tests found higher-than-acceptable levels of nitrosamine chemicals.
Manufacturers also recalled different hypertension medications after they were found to contain high levels of nitrosamine impurities.
Chantix was lauded in the early days of its use by public health officials for its ability to help curb smoking addiction.
In 2009, however, safety concerns prompted the FDA to require a warning label that Chantix could cause psychiatric side effects. In 2016, the FDA stopped requiring the warning.
Sales of Chantix reached $919 million in 2020, representing a 17% drop from 2019. It lost U.S. patent protection almost a year ago, leading to the generic competition.
According to a Pfizer spokesperson, no cancer-related safety issues have been detected in the testing of Chantix, or with people taking the drug.