FDA Warns of Contaminated At-Home COVID-19 Tests
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
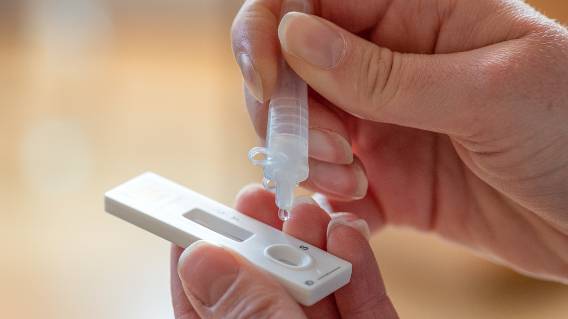
U.S. health officials announced a recall earlier this month of more than 500,000 COVID-19 at-home tests after the liquid used in the kits was found to be contaminated with bacteria.
SD Biosensor Inc., the maker of Pilot COVID-19 At-Home tests, issued the voluntary recall on May 4 and urged consumers and health care providers to check lot numbers and throw away any contaminated tests.
Roche Diagnostics distributed the tests nationwide. About 500,000 kits were sent to CVS Health and another 16,000 tests were stocked by Amazon. Officials are working to determine how many of those tests were sold. Any remaining tests were pulled from shelves.
The contamination impacted 44 lots. A complete list of lot numbers can be found in the FDA recall notice. None of the potentially contaminated kits were distributed by the federal government’s testing program.
Routine Quality Checks Reveal Bacteria
The South Korea-based manufacturer discovered the contamination during routine quality testing, according to information on Roche’s website. A spokeswoman for SD Biosensor told CBS News in a statement that the contamination may have stemmed from a raw materials supplier. The company ramped up its quality control efforts and “cut off” the supplier behind the contamination, CBS reported.
Bacteria was found inside the liquid in the individual pre-filled, ready-to-use sealed tubes.
“The liquid solution provided in the affected Pilot COVID-19 At-Home Test kits has been found to be contaminated with organisms such as Enterococcus, Enterobacter, Klebsiella and Serratia species,” the FDA wrote in a notice.
Those bacteria could cause a wide range of issues, including infections to the urinary tract, bloodstream or respiratory tract. Some strains have developed antibiotic resistance, making them harder to treat. People with weakened immune systems may be more susceptible to illness. Consumers who used a contaminated test may also have been issued a false positive or false negative result.
How to Dispose of Contaminated Tests
The FDA asks consumers to throw out the entire recalled test kit, keeping it sealed. Do not pour the liquid out in the drain.
“Direct exposure to the liquid in the tube through misuse or spillage could potentially lead to serious illness,” the agency warned.
Users could accidentally touch the liquid while opening the tube, during testing or while discarding a used test. If the liquid in the tube comes into contact with skin or eyes, flush the area with water. Medical attention should be sought if the area becomes irritated, the FDA said.
Signs of bacterial infection could include fever, discharge, red eyes and more. Anyone with symptoms after touching the contaminated test liquid should seek medical attention. Spilled liquid should be cleaned up with a disinfectant and hands washed with soap and water, according to Roche.
So far there have been no reports of injuries or deaths linked to the contaminated tests, according to the FDA. To report an incident, visit the agency’s MedWatch adverse event page.
Consumers who would like a replacement test can contact Roche online or call 1-866-987-6243 and select option 1.