NuvaRing Side Effects
Common NuvaRing side effects include headaches, vaginitis and breakthrough bleeding. This hormonal contraceptive ring can cause blood clots and other cardiovascular complications, especially among users over 35 or who smoke. A boxed warning indicates this risk.
Article Continues Below
Board-certified physicians medically review Drugwatch.com content to ensure its accuracy and quality.
Drugwatch.com partners with Physicians’ Review Network Inc. to enlist specialists. PRN is a nationally recognized leader in providing independent medical reviews.
Reviewer specialties include internal medicine, gastroenterology, oncology, orthopedic surgery and psychiatry.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Common Side Effects
- Headaches, breakthrough bleeding, device complications and vaginal infections
- Serious Side Effects
- Deep vein thrombosis, anxiety, gallstones and vomiting
- Boxed Warning
- Smoking increases the risk of cardiovascular effects when using NuvaRing.
Most Recent Side Effects Information
As of June 30, 2024, pulmonary embolism and deep vein thrombosis (DVT) were the most common NuvaRing side effects reported to the Food and Drug Administration. Reports also frequently listed breakthrough bleeding and incorrect use of the product.
The FDA considered 47.5% of the reports it received to be serious adverse events.
| FDA Adverse Event Reports for NuvaRing Side Effects | |
|---|---|
| Total cases reported | 19,407 |
| Serious cases (including deaths) | 9,221 |
| Deaths | 251 |
Disclaimer: Reports sent to the FDA don’t necessarily mean the drug caused an adverse event. Consult a health care professional before stopping or changing medication.
When used correctly, NuvaRing appears to have a strong safety profile. However, you should always discuss the possible side effects and risks with your health care provider.
FAERS data indicate that adverse effects from a failure to follow the prescribed regimen were widespread among NuvaRing users. Improper use can cause vaginal irritation, injury and infections. It can also lower NuvaRing’s effectiveness.
Common Side Effects
According to NuvaRing’s prescribing information, the most common reactions leading to study withdrawal were device-related events, including expulsion or discomfort, mood changes, headache and vaginal symptoms. It further states that 13% of women enrolled in clinical trials withdrew because of side effects.
Some NuvaRing users reported broken devices that can cause vaginal injury. Additionally, sexual partners may develop penile disorders, such as rash, from contact with a vaginal ring.
- Vaginitis (13.8%)
- Headache, including migraine (11.2%)
- Mood changes (6.4%)
- Expulsion or discomfort (6.3%)
- Nausea or vomiting (5.9%)
- Vaginal discharge (5.7%)
- Weight gain (4.9%)
- Vaginal discomfort (4.0%)
- Breast pain or tenderness (3.8%)
- Dysmenorrhea (period pain) (3.5%)
- Abdominal pain (3.2%)
- Acne (2.4%)
- Decreased libido (2.0%)
Many common NuvaRing side effects, such as expulsion and discomfort, relate to how the patient inserted it. Other side effects, such as headache, weight gain and breast tenderness, are similar to the side effects of other types of hormonal birth control.
| Side Effect | Description |
|---|---|
| Toxic shock syndrome | Users have reported TSS, though cases are very rare. Some of the women were also using tampons. |
| Liver disease | Liver function may be disrupted. Liver tumors called hepatic adenomas may burst, causing potentially fatal bleeding, and long-term use can increase the risk of certain liver cancers. |
| High Blood Pressure | An increase in high blood pressure has been reported by women using CHCs. This risk increases with age. It can result in stroke, heart failure heart attack and kidney failure. |
| Headaches | Women who experience new, more frequent, severe headaches while using the contraceptive should consult their doctor. |
| Uterine bleeding | Unscheduled bleeding may occur with users of CHCs in the first three months of use. If this persists beyond three months of use, you should talk to your doctor. |
| Amenorrhea or Oligomenorrhea | Infrequent periods or lack of a scheduled period while using the vaginal ring may indicate pregnancy. |
Mild side effects tend to be most pronounced when first starting NuvaRing. They usually lessen and may go away completely after a few months of use.
Talk to your doctor if side effects worsen, interfere with your ability to use NuvaRing or last for more than a few months.
Rare but Serious NuvaRing Side Effects
NuvaRing users reported severe adverse effects including blood clots, heart attack and stroke. Cardiovascular side effects can lead to serious health consequences, including death.
One 2017 study published in the Journal of Stroke and Cerebrovascular Diseases concluded that vaginal contraceptive rings led to a 2.5 times increased stroke risk.
Seek emergency medical care if you experience any of the following severe side effects.
- Blood Clots
- Stroke
- Toxic shock syndrome
Post-marketing experience shows that hypersensitivities, including allergic reactions leading to anaphylaxis, are possible with NuvaRing. Anaphylaxis is a life-threatening condition.
Notify your doctor immediately if you develop hives, difficulty breathing or swelling of the face, throat or nose, as these could be symptoms of anaphylaxis.
Blood Clots
A 2022 report in the American Journal of Case Reports showed an approximately three-fold increase in thromboembolism among NuvaRing users compared to individuals who do not use hormonal birth control methods. This is a blood clot that breaks loose from where it formed and becomes lodged in another body part. The rates of thromboembolism in NuvaRing users averaged .08%, whereas the rate was .03% in the control group.
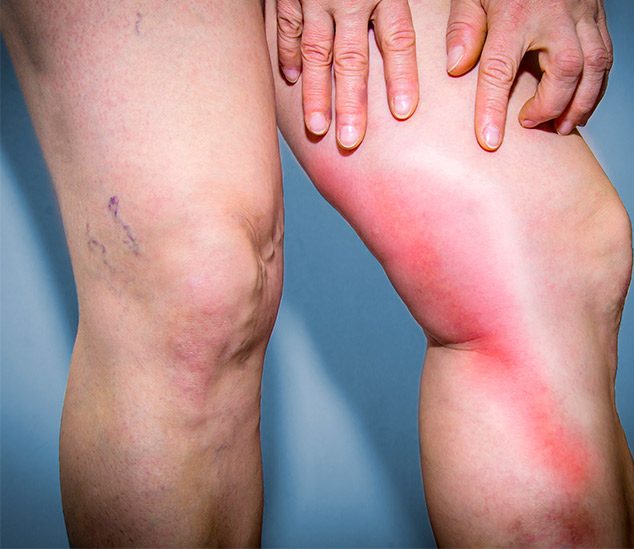
Blood clots can cause serious injury, including death. There are various symptoms of blood clots which depend on where the clot is. With NuvaRing, the clots are typically deep vein thrombosis clots. This type typically forms in the leg and causes swelling and pain.
Blood clot symptoms include any new and unexplained limb pain – especially in the calf -, severe headaches, breathlessness, yellowing of the skin or vision problems.
Case Study: Jamila Williams’s NuvaRing Blood Clot Side Effects
Court records show that 26-year-old Jamila Williams from Cincinnati, Ohio, began using the NuvaRing contraceptive in August 2014. After just two months, in October, she started feeling pain in her lower ribs. Concerned about her health, she went to Mercy Health for medical help.
Diagnosis of Pulmonary Embolism
On Oct. 26, 2014, a CTA scan showed that Williams had developed severe blood clots in her lungs. She required immediate hospitalization, where she was put on medication to prevent more clots. Her doctor also told her to stop using the NuvaRing.
Ongoing Health Challenges
Williams filed a lawsuit claiming that using NuvaRing caused ongoing health issues for her. Her recovery required multiple doctor’s appointments, check-ups and medications.
NuvaRing Lawsuits
In February 2014, NuvaRing manufacturer Merck agreed to a $100 million deal to settle multiple NuvaRing lawsuits. Plaintiffs claimed Merck knew of the risk of blood clots related to product use but failed to provide patients with a clear warning about the risk.
In total, over 3,500 plaintiffs took advantage of the settlement. Some cases continue to play out individually, but most have been resolved.
NuvaRing FDA Warnings
NuvaRing has a boxed warning for the risk of cardiovascular side effects among individuals who smoke. This is the strictest type of FDA warning, indicating a drug may cause serious health consequences or death.
“Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive (CHC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.”
The warning also indicates that women over 35 and those who smoke should not use combined hormonal contraceptives (CHCs). This includes treatments such as NuvaRing and hormone-based patches and pills.
Other FDA Warnings
The manufacturer updated NuvaRing packaging information in 2024 to warn of an increased risk of developing elevated liver enzymes in patients using NuvaRing who are also on certain hepatitis medicines.
This comes after the addition of two warnings in 2018. One warned of how there have been reports of vaginal injury with ring breakage. According to the warning, ring breakage is more likely to happen if the patient inserts the device incorrectly. The other warning was for the risk of hypersensitivity reactions.
The prescribing information also contains a warning highlighting the risk of pregnancy associated with using NuvaRing in a way contrary to the provided information. It details instructions for replacing an expelled device depending on the timing of your menstrual cycle and how long the ring was out.
Some patients using NuvaRing reported allergic reactions of anaphylaxis and swelling. Prescribing information recommends discontinuing the use of the device and seeking medical treatment for these conditions.
Anyone with a known hypersensitivity to a component or ingredient in NuvaRing should not use it.
Known Risk Factors
Some lifestyle choices, medications and health conditions increase the risks of NuvaRing side effects. According to its prescribing information, people who smoke, are over the age of 35, have unexplained vaginal bleeding or take certain hepatitis C drug combinations should not use NuvaRing.
- Regular smoking
- Over age 35
- History of blood clotting disorders
- Heart conditions, including: stroke, heart attack, high blood pressure, & heart valve problems
- History of breast or other hormone-sensitive cancers
- Migraine with aura, numbness or vision changes
Sharing a complete overview of your medical history and lifestyle with your health care provider before using NuvaRing can help reduce the risk of side effects. This allows you to work together to make an informed decision about safe and effective contraception.
NuvaRing Alternatives
NuvaRing can be an effective contraceptive method for many people, but it does pose some health risks. If you are concerned about possible side effects, you might want to consider alternative forms of contraception.
However, many NuvaRing alternatives also contain hormones that can cause similar side effects.
- Birth control pills, implants and patches
- Condoms (male and female)
- Intrauterine contraceptive devices (IUD)
As with any medication, you must weigh the benefits and risks of using a specific contraceptive. For example, IUDs can be hormonal or non-hormonal, which influences the side effects.
Ease of use may also play a factor in your decision. You only need to insert NuvaRing once per month, and IUDs last several years, as opposed to taking a daily pill or using barrier contraceptives for sexual interactions.
However, IUDs and implants require a doctor for insertion and removal, whereas patients insert vaginal rings or apply patches at home.
You can discuss any concerns with your health care provider to find a solution you are comfortable with.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


