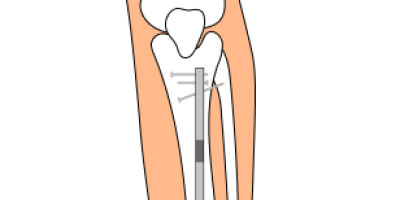
NuVasive Precice System Lawsuits
NuVasive issued a recall for its limb lengthening Precice system implant over biocompatibility concerns in February 2021. People have filed lawsuits for injuries related to biocompatibility problems such as bone abnormalities, tissue degradation and bone degradation.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by Wilson & Peterson LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Cassandra L. Sundblad, Esquire
- Last update: April 15, 2025
- Est. Read Time: 4 min read
Why Have People Sued NuVasive?
People sued NuVasive because the company’s Precice devices could lead to biocompatibility issues. Biocompatibility is a measure of how well an implant does in the body without causing rejection or other problems. Bio-incompatibility may cause tissue reactions, inflammation, immune responses, cancer and other negative reactions.
On July 8, 2021, the Food and Drug Administration issued a letter to health care providers warning them of potential biocompatibility concerns with Precice devices. The FDA received reports of pain, changes in soft tissue and changes in the bone surrounding the stainless steel Precice Stryde device.
So far, the agency hasn’t received reports of problems with Precice implants made from titanium. In December 2021, the FDA advised the public that the benefits for patients using titanium Precise devices outweigh the risks compared to alternative treatments.
In March 2023, the FDA announced that NuVasive received 510(k) clearance for the “expanded use of the Precice IMLL system (including Precice Short) for limb lengthening of the shin bone (tibia) and thigh bone (femur) in patients greater than 12 years old.” The agency has not received any reports of adverse events tied to titanium-based devices, though they continue to monitor them.
A of December 2025, there have been no major new developments in this litigation. Drugwatch’s legal partners are currently not taking NuVasive Precice lawsuit cases.
Which Precice Devices Have Been Recalled?
NuVasive pulled all Precice stainless steel devices from the U.S. market in February 2021 because of side effects such as pain and bone changes in patients using the Precice Stryde device. The problems seem to occur where the telescoping (moving) segments of the nail come together, according to NuVasive. However, the company hasn’t confirmed the exact cause for the adverse events.
The company also initiated a recall for the titanium Precice devices for biocompatibility testing in February 2021. They did not withdraw them from the market at that time, but they did place a shipping hold on titanium Precice devices in April 2021. That shipping hold was officially lifted in November 2021.
In addition, the CE certification for the company’s MAGEC and Precice systems in Europe was suspended and all sales of the device have been put on hold.
“At this time, the FDA is uncertain if the root cause of these adverse events is due to the stainless-steel material or related to design features and materials common to all Precice devices.” — FDA”
Stainless Steel
Patients with stainless steel Precice devices have reported pain and changes in surrounding bone and soft tissue. According to the FDA, these side effects may be related to corrosion, wear, and previously unanticipated exposure of components.
In the FDA’s letter to health care providers, the agency noted that it is working to evaluate new testing to address biocompatibility issues. NuVasive and the FDA are working together to collect more data that can provide insights into the causes of the complications and to make sure patients who currently have a Precice device are properly monitored.
- Precice Bone Transport
- Precice Plate
- Precice Stryde
At this time, the FDA is not recommending the early removal of devices in people who are not experiencing any reactions to their current stainless steel Precice devices. The agency does urge patients and their doctors to check for changes “during routine radiographic monitoring” and pay close attention to any unexpected symptoms, such as pain.
Titanium-based
So far, the FDA hasn’t received reports of biocompatibility issues stemming from titanium-based Precice devices. NuVasive is currently investigating these devices for potential connections to the adverse events seen with stainless steel devices.
- Precice Freedom
- Precice Intramedullary Limb Lengthening (IMLL) Device
- Precice Short
- Precice Unyte
While titanium is known for its favorable biocompatibility, implants made with this metal also have some disadvantages, according to a 2020 study published in Materials.
“These include low biologic activity, which reduces the growth of fibrous tissue and allows loosening of the prosthesis, the possibility of metallosis and related inflammation or other allergic reactions, as well as abrasion of the material during operation,” authors write.
On Dec. 1, 2021, the FDA released an update on titanium-based devices that had been removed from the market as a precaution. On Nov. 30, 2021, the ship hold for these devices was removed.
“The FDA believes it is in the best interest of patients to make titanium-based Precice devices available in the United States. At this time, the overall benefits of the devices outweigh the known risks for on-label use with the updated labeling, compared to alternative treatments,” the agency said.
Injuries Named in Lawsuits against NuVasive
Injuries related to biocompatibility are the main focus of Precice lawsuits, these include tissue reactions, bone problems and other complications stemming from the body reacting poorly to the implant.
For example, one 2021 study published in Acta Othropaedica noted that patients with Stryde implants had pain and abnormal bone growth at the nail junction. Researchers found 20 out of 23 retrieved nails had corrosion and discoloration. They also found biological material inside the nail.
Study authors concluded the design of the screw holes and locking screws may have contributed to the patients’ health problems.
NuVasive is investigating potential health problems, such as cancer, chronic toxicity, developmental toxicity and reproductive toxicity, linked to biocompatibility problems with its Precice devices.
- Bone abnormalities
- Bone degradation
- Cancer
- Developmental effects
- Pain
- Reproductive system problems
- Skin irritation or burns
- Tissue death (necrosis) around the implant
- Thrombosis
- Tissue degradation
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.