NuVasive Precice System Side Effects
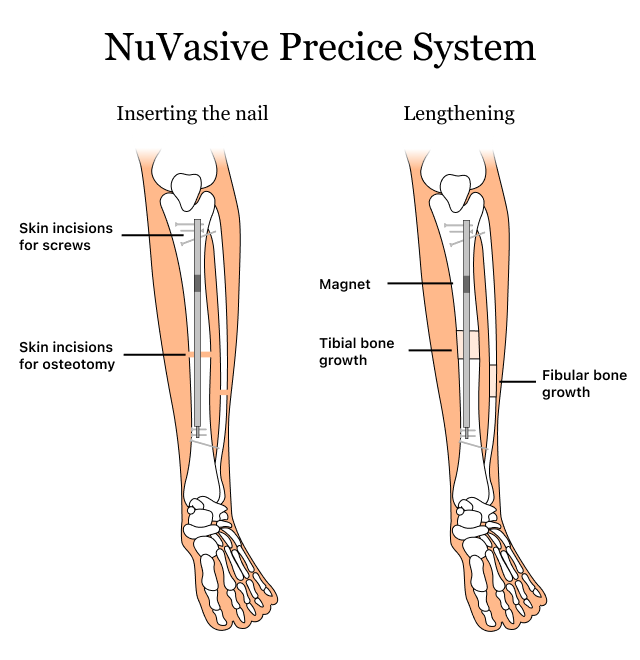
NuVasive Precice System devices have been linked to adverse events such as biocompatibility issues, device breakage and insufficient bone lengthening. These NuVasive Precice complications may cause pain and abnormal growth. Sometimes, additional surgeries are required.
Article Continues Below
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Serious Side Effects
- Biocompatibility problems such as tissue degradation, bone degradation and bone abnormalities
- Most Common Side Effects
- Device breakage and insufficient lengthening
- Average Complication Rate In Studies
- 31% complication rate per segment lengthened
Latest Side Effects Information for NuVasive Precice Devices
According to the U.S. Food and Drug Administration’s MAUDE Database, there have been reports of biocompatibility issues, device breakage and insufficient bone lengthening in regard to the NuVasive Precice bone-lengthening medical device.
A device’s biocompatibility is its ability to work with your body without harming your tissues or organs. The biocompatibility issues of NuVasive Precice devices can cause pain and abnormal growth at the implant site. Additionally, device breakage is a common side effect of stainless steel Precice implants. NuVasive has recalled some Precice devices, such as Precice Stryde, Precice Plate and Precice Bone Transport, due to these issues.
NuVasive urges healthcare providers to report any adverse events related to its Precice devices through the FDA’s MedWatch program.
Study Finds Complications With One-Third of Precice and FitBone Devices
Researchers writing in the Journal of Acta Orthopaedica found complications in about one in three lengthening procedures.
They reviewed 41 studies involving 782 patients who used these nails to lengthen 983 leg segments. The complications varied. Some were minor and needed little treatment, while others were serious and caused lasting health problems. In some cases, the complications were severe enough to cause a major change in the treatment plan, such as changing the lengthening goals.
The most common problems were related to the device itself or the bone.
The study found that while motorized nails are a newer and more comfortable alternative to older methods, such as external fixators, they are not without risks. They concluded that complications with motorized nails are common.
Serious Side Effects of NuVasive Precice Devices
The NuVasive Precice devices have been associated with several severe side effects. Patients have reported pain, abnormal bone growth and device breakage. The devices have encountered problems such as loosening, fracturing, migration and corrosion.
Both NuVasive and the FDA say these complications can cause chronic pain and may require more surgeries. The FDA has recommended the removal of these devices after one year to minimize risks.
The FDA and NuVasive recommend that doctors consider alternative treatments for patients who need bone lengthening.
Case Study: Complications with NuVasive Precice Stryde Device
To correct her leg length discrepancy, Alyssa Osos of Michigan underwent surgery to receive the NuVasive Precice Stryde limb lengthening device. The device was made from BioDur 108 stainless steel.
Health Issues
After the implant, Osos had severe side effects, including heavy menstrual bleeding and pain in her reproductive organs. An ultrasound revealed a large ovarian cyst. Her condition worsened when she also developed chromium toxicity and toxic chemicals in her liver.
Device Failure
crutches to prevent stress fractures. She lost months of physical therapy progress and experienced significant discomfort.
Surgical Revision
Osos underwent revision surgery to remove the failed device, but x-rays showed leftover fragments around the implantation site. Follow-up appointments confirmed bone damage, chromium toxicity and ongoing health complications.
Bone Lengthening Risks of NuVasive Precice Devices
While bone lengthening surgery can fix limb length differences, it has serious risks. Bone lengthening devices like the NuVasive Precice system, which uses adjustable rods with internal magnetic mechanisms, have specific concerns.
- Infection and Poor Healing
- One major risk is infection. This often occurs at the site where the device is implanted or where external pins enter the skin. Additionally, bone healing can be unpredictable and delayed. Sometimes, a nonunion, meaning the bone fails to heal, occurs.
- Nerve and Blood Vessel Damage
- During surgery, there is a risk of damaging nearby nerves or blood vessels. This can cause pain, numbness or even loss of function in the affected limb.
- Pain and Discomfort
- Many patients have some pain and discomfort during the lengthening process and recovery period. This can be managed with pain relief, but it may still impact daily activities.
- Device-Related Issues
- NuVasive Precice devices have faced scrutiny over their biocompatibility. This is especially true for their stainless steel models, which were recalled. Investigations into the titanium devices are ongoing.
- Complications from Surgery
- Like with most surgeries, there are risks for allergic reactions to anesthesia, blood clots and breathing. Repeated surgeries may be necessary to adjust or remove the devices, increasing the risk of complications.
- Long Recovery Period
- Recovery from bone lengthening surgery can be lengthy, requiring ongoing physical therapy and regular follow-ups. The consolidation phase, where new bone hardens, can take several months, especially in adults.
Patients considering bone lengthening surgery should discuss these risks with their healthcare provider. Regular monitoring and adherence to post-surgery care are crucial to minimize complications.
Side Effect Safety Communications
In response to several adverse events, the company and the FDA took significant steps to ensure patient safety. Since February 2021, there have been several notable safety-related communications and actions. These include a safety notice about long-term toxicity and an urgent recall.
A more positive communication included an FDA letter that approved more uses for the Pęcice Intra-medullary Limb Lengthening (IMLL) system in patients over 12 years of age.
NuVasive Alerts Doctors With a Field Safety Notice
On Feb. 1, 2021, the company issued a safety notice to alert health care providers about problems with some Precice medical devices.
NuVasive told doctors that the devices did not meet safety standards for long-term toxicity risks, such as cancer and developmental and reproductive side effects.
The company said not to use the devices for the time being and gave instructions on how to return them if they had already been sent out.
NuVasive Issues Urgent Recall Notification
On Feb. 20, 2021, NuVasive Specialized Orthopedics issued a recall notification for Precice Stryde, Precice Plate, Precice Bone Transport devices.
This recall was due to reports of pain and unusual bone issues where the different parts of the device fit together.
The initial investigations suggested that the issues weren’t related to biological safety. Regardless, the company recalled the devices to investigate their safety.
FDA Issues Letter to Doctors
In June 2023, the FDA told doctors they could use the Precice Intra-medullary Limb Lengthening (IMLL) system for patients over 12 in more ways than before.
The agency also mentioned that it was still looking into whether some Precice devices made with stainless steel are safe. Some of these devices are not available in the U.S. because of concerns about how well they work with the body.
What Should You Do If You Have a Precice Implant?
If you have a NuVasive Precice device implanted, you can consult your healthcare provider for a thorough assessment. Following these steps may help ensure your safety:
- Monitor symptoms: Watch for pain, abnormal body changes, or any signs of adverse effects and report them to your doctor immediately.
- Consult your physician: Regular check-ups are essential. Discuss your specific case, especially if you have biocompatibility concerns.
- Follow instructions for use: Adhere strictly to post-operative care guidelines. This includes limiting weight-bearing activities and scheduling the device’s removal after one year.
- Seek alternative treatments: If you are experiencing significant issues, your healthcare provider might suggest alternative treatment options. The appropriate alternative treatment depends on your condition. For example, for children with limbs of different lengths, epiphysiodesis and hemiepiphysiodesis are potential treatments. These treatments temporarily or permanently stop the growth of the longer limb so the shorter one can catch up.
- Report adverse events: You should report any adverse reactions or quality problems to your doctor as soon as possible. Afterward, you can report to the FDA's MedWatch Adverse Event Reporting program.
- Talk to an attorney: If you’ve been injured by a Precice device, you may be entitled to compensation. Consulting with a personal injury attorney may help you learn if you qualify to file a NuVasive Precice lawsuit.
Your healthcare provider can guide you based on your individual health needs and the latest safety information.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


