Paragard Lawsuit
Paragard lawsuits claim that the Paragard IUD can break upon removal and that the manufacturer failed to adequately warn the public. Claims from all over the country have been transferred to federal multidistrict litigation in Georgia to streamline proceedings.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Trent B. Miracle, Esquire
- Last update: February 5, 2026
- Est. Read Time: 6 min read
- Number of Paragard Cases:
- There are 3,867 lawsuits pending as of February 2026.
- MDL 2974:
- Judge Leigh Martin May is the judge for MDL 2974, and the litigation is centralized in the U.S. District Court for the Northern District of Georgia.
- Bellwether Dates:
- Test trials are scheduled for January, March and May 2026.
Am I Eligible To File a Paragard Lawsuit?
According to our legal partners, you may qualify to file a Paragard IUD lawsuit if your Paragard IUD broke upon removal, and you experienced serious injury or Paragard side effects.
- Documented breakage on removal
- Imaging that shows retained fragments
- Medical intervention (attempted removal or surgery)
- Records of symptoms or complications
- Statute of limitations hasn’t passed
Women who filed lawsuits claim that Paragard broke during removal, leaving pieces of the IUD in their bodies. Some women required surgery to remove the device and treat complications.
According to CooperSurgical’s website, “Paragard removal is nonsurgical and done by a health care provider during a routine office visit in just a few minutes.” The arms of the device should fold up when removed, according to the instructions for removal included in the prescribing information.
- Infection
- Infertility
- IUD pieces breaking and getting lodged in organs
- IUD pieces left in the body, causing infections and allergic reactions
- IUD pieces that cannot be removed from the body
- IUDs shifting position
- Need for surgical intervention, such as laparoscopy or laparotomy and hysterectomy
- Pain
- Perforation of the cervix or uterus
If you experienced an injury because of Paragard IUD removal, you may qualify to receive compensation for pain and suffering, medical bills or lost wages. Before moving ahead with litigation, it is important to speak to a lawyer to review your options and the specifics of your case.
What Evidence To Gather
Before you speak to an attorney about filing a Paragard IUD lawsuit, you should prepare by gathering evidence to strengthen your case. Evidence may include medical records and proof of financial and emotional damages you suffered.
- Damages:
- Bills, wage loss, symptom diary.
- Device Info:
- Device lot details (if available), photos of retrieved pieces.
- Imaging:
- Ultrasound, X-ray or CT showing fragments.
- Medical Records:
- Insertion/removal notes, operative reports for surgical removal (hysteroscopy/laparoscopy).
Make sure to speak to your attorney about additional evidence you may need. They can also help you gather some of your medical records.
Why Are Women Filing Paragard IUD Lawsuits?
The Paragard IUD lawsuits claim a design flaw led to the IUD device breaking during removal. This meant that part of the device could remain lodged in the uterus or other internal organs, causing injuries.
The women claim that the manufacturers failed to warn doctors and patients of the risks. As a result, plaintiffs suffered several debilitating effects from undergoing Paragard IUD removal. Some women required surgery as a result.
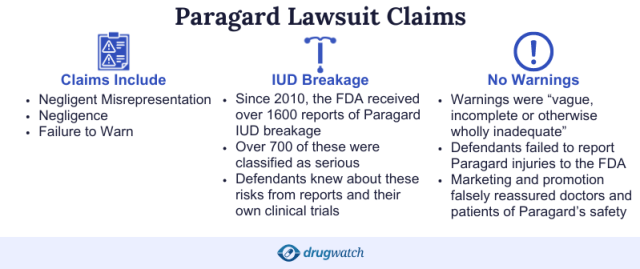
“This litigation involves allegations that the Paragard intrauterine device (IUD) has a propensity to break upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain,” according to the Northern District of Georgia’s MDL page.
- IUD has manufacturing and design defects.
- Labeling doesn’t adequately warn about breakage risk.
- The manufacturers, CooperSurgical and Teva Pharmaceuticals, were negligent.
In December 2020, the Judicial Panel on Multidistrict Litigation consolidated dozens of lawsuits across the nation in the Northern District of Georgia under Judge Leigh Martin May.
“This litigation involves allegations that the Paragard intrauterine device (IUD) has a propensity to break upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain.”
Examples of Paragard Lawsuits
Paragard manufacturer CooperSurgical continues to make claims supporting the device’s safety and benefits. However, several women have already filed lawsuits after suffering injuries upon the removal of their IUD.
Medical researchers investigating IUD side effects have also recommended improved protocols when removing Paragard IUDs.
Georgia Bowers v. Teva et al.
Georgia Bowers filed her suit against Teva and CooperSurgical in September 2020. She received her Paragard in January 2017. In September 2017, she visited her doctor to have the IUD removed.
An ultrasound revealed the device was incorrectly positioned. Bowers’ doctor followed instructions for removing the IUD, but only part of it came out. One arm was missing. Bowers’ doctor tried to remove the broken piece by colposcopy but failed.
“Defendants had knowledge that there was a significant increased risk of adverse events associated with Paragard IUD, including arm breakage, and despite this knowledge … Defendants continued to manufacture, market, distribute, sell and profit from sales of Paragard IUD.”
“At all relevant times, the Teva Defendants had knowledge that there was a significant increased risk of adverse events associated with Paragard IUD, including arm breakage, and despite this knowledge the Teva Defendants continued to manufacture, market, distribute, sell and profit from sales of Paragard IUD,” Bowers’ lawsuit said.
Carley Tredway v. Teva et al.
Carley Tredway filed her suit against Teva and CooperSurgical in September 2020. Tredway received her Paragard in 2008. In 2018, she went to get her Paragard removed.
“Plaintiff and her doctors were provided with no warning from the Defendants of the risk of Paragard IUD failure and injury, nor were Plaintiff and her doctors provided with adequate warning of the risk of removal of ParaGard IUD.”
Her doctor followed the instructions to remove the IUD, but one arm remained in the uterus upon removal. Tredway’s doctor removed the remaining arm via hysteroscopy a month later.
“Prior to her procedures, Plaintiff and her doctors were provided with no warning from the Defendants of the risk of Paragard IUD failure and injury, nor were Plaintiff and her doctors provided with adequate warning of the risk of removal of ParaGard IUD,” Tredway’s complaint said. “This information was known or knowable to the Defendants.”
Paragard Lawsuit Settlement Amounts
The Paragard litigation is in progress, and as of February 2026, there has been no global settlement. If an out-of-court settlement isn’t reached, the first trials may begin in 2026. The result of these first trial cases will likely influence possible settlement amounts.
Prior IUD birth control cases, like the Mirena IUD settlement, can provide some indication of lawsuit settlement amounts, but this is only a guess, and you shouldn’t base any potential settlement amounts on this figure.
In August 2017, Bayer offered $12.2 million to settle 4,600 lawsuits after women successfully sued the manufacturer for claims that their IUD moved around in the body and punctured or injured organs.
If you have questions about any potential settlement amounts, make sure you speak to your lawyer directly.
Settlements can sometimes be reached before a trial begins or once it is underway. Monetary settlements for damages can vary significantly based on the details of an individual case. Settlement talks generally start once the first few test cases go to trial and juries award verdicts.
Expert and Patient Perspectives on Paragard Lawsuits
Latest Paragard Lawsuit Updates
Litigation involving the Paragard intrauterine copper contraceptive device is ongoing. Our legal partners are actively accepting new cases on behalf of women who experienced pain, infertility and other injuries from broken Paragard IUD pieces embedded in their organs.
As of February 2026, there were 3,867 pending lawsuits and a total of 4,139 cases filed against the company in federal court in Georgia under MDL 2974.
The first bellwether trial is expected to begin in January 2026.
From our legal research through court documents and talking to our legal partners, we’ve compiled a timeline of the biggest developments in this litigation below.
-
February 2026:
In a significant blow for these cases, Teva has prevailed in the first Paragard bellwether trial. This makes the next two bellwether trials critical for people who have filed lawsuits. Wins for plaintiffs in those cases could be a significant development for thousands of cases.
-
January 2026:
The judge overseeing the Paragard lawsuits has granted summary judgment to CooperSurgical in the bellwether cases. This is a legal procedure where the judge rules for one side without holding a trial. This does not mean that the litigation has failed. CooperSurgical only acquired the NDA to Paragard in 2017, which was after the bellwether plaintiffs received their implants. Teva Pharmaceuticals remains a defendant.
Teva's separate summary judgment motion was not fully successful, meaning that the bellwether cases are likely cleared to proceed to trial. This is a positive development for people who have filed lawsuits. -
December 2025:
With bellwether trials right around the corner, there are now 3,749 active Paragard lawsuits in federal court. The judge overseeing these cases recently trimmed some of the claims of these lawsuits on summary judgment, dismissing design defect claims. The judge essentially determined that Paragard can't be blamed for a defective design since it wasn't the NDA holder when the IUDs were placed in the bellwether cases. The claims that are more critical to these lawsuits are that Paragard failed to warn customers of a risk connected to their product, and those claims remain in place.
-
November 2025:
The Paragard litigation has grown by 63 new active cases over the last month as work continues in the MDL. These cases are still progressing toward bellwether trials, which can play a key role in influencing settlement talks for all active lawsuits.
-
October 2025:
The number of active Paragard lawsuits continues to grow, with 3,595 active cases in the MDL this month. Key deadlines for the first bellwether trial are fast approaching, with dates set throughout November and December for various pretrial milestones. That first trial, which could have a key impact on the litigation, remains set for January.
-
June 2025:
A new scheduling order has provided a better look at when the first Paragard lawsuits will go to trial. As anticipated, the first bellwether trial will get underway in January 2026. A second bellwether trial will follow in March, with a third one set for May. The outcome of these trials can play a massive role in the result of many lawsuits, with their results likely to be key to settlement negotiations.
-
May 2025:
The first bellwether trial case has been selected in the Paragard MDL. After reviewing possible options submitted by both sides, the judge overseeing the litigation has selected a case that was suggested by the defendants. The first trial is slated to begin in January 2026.
-
February 2025:
In a blow to plaintiffs, the judge overseeing the Paragard lawsuits pending in federal court opted to dismiss claims from several states over statute of limitations issues. The issue in question was pinpointing when plaintiffs’ injuries occurred and when exactly the clock starts on filing a claim. The court essentially determined that injuries took place while the device was still in plaintiffs’ bodies. “It would defy logic to find that a broken Paragard may not have inflicted medically ascertainable injuries until after it had already been removed from the woman’s body,” the judge stated.
Also in February, the start of the Paragard bellwether trials was once again delayed. The first trial was now set to begin Jan. 12, 2026. -
October 2024:
The court scheduled two bellwether trials to begin Dec. 1, 2025, and Feb. 2, 2026, pending availability of defendants' counsel.
-
August 2024:
No new developments happened in August in the multidistrict litigation. In past months, Judge Leigh Martin May replaced some members of the Plaintiffs’ Steering Committee and discovery continued to move forward slowly, according to our review of the MDL docket.
-
July 2024:
The next status conference was scheduled for the end of July.
-
December 2023:
Several cases joined the MDL and conditional transfer orders were filed.
-
September 2023:
Judge Leigh Martin May outlined the protocols for depositions for bellwether pool plaintiffs in October 2023. The first trial was expected to be on or before Oct. 28, 2024, according to the judge’s order.
-
February 2023:
The judge outlined the process for selecting 10 cases to be the first group of bellwether test trials to go to trial in 2024.
-
January 2023:
Judge M. Gino Brogdon Sr. (retired) was selected to serve as a settlement mediator. We viewed the progress as a sign parties were negotiating a global Paragard settlement and expected talks to take place throughout 2023.
Paragard bellwether trials are called test trials because the outcome of these cases will help lawyers on both sides better evaluate a global settlement value.
In some cases, defendants will settle a case ahead of trial to avoid potentially paying more damages and risking a jury verdict.
Paragard Recall Status and Manufacturer
As of February 2026, there has been no Paragard IUD recall despite reports and complaints about device breakage. The FDA and manufacturer CooperSurgical haven’t released any warnings or safety communications related to device breakage.
CooperSurgical and former Paragard manufacturer Teva Pharmaceuticals are defendants in this litigation. Both companies appear in complaints because CooperSurgical is the current manufacturer, and it bought Paragard from Teva in 2017.
Both companies are still liable for any potential design issues, and neither company warned about the potential for device breakage.
Paragard Lawsuit Frequently Asked Questions
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.








