Philips CPAP
Philips is the largest maker of continuous positive airway pressure machines in the world. CPAP machines are used to treat obstructive sleep apnea and may have benefits for other health conditions as well. Side effects are possible. Philips recalled some DreamStation CPAP devices in June 2021 and announced in January 2024 that it would no longer sell its CPAP or BiPAP machines for sleep apnea in the United States.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by MaryAnn DePietro, CRT
- Last update: December 1, 2025
- Est. Read Time: 6 min read
What Does a CPAP Machine Do?
Health care providers prescribe the Philips CPAP and other brands of machines to help people with sleep apnea breathe better when sleeping. Sleep apnea is a condition where a person stops and starts breathing repeatedly while they sleep.
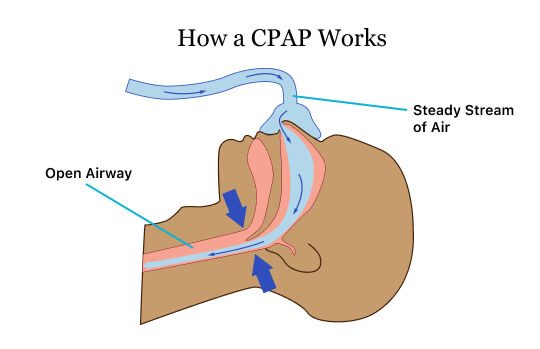
CPAP devices take air from the room, pressurize it and push it through a tube to a mask a person wears on their face. The pressure settings can be adjusted depending on what each individual needs.
Each brand of CPAP may have slightly different features and designs. Some newer machines are available in smaller travel sizes and come with mobile apps that collect data about device use and sleep quality.
Philips DreamWear CPAP masks are “designed to feel like you’re not wearing a mask at all,” according to the company’s website. They have an open-face construction and a flexible over-the-head frame.
How Does a CPAP Machine Treat Sleep Apnea?
In order to treat sleep apnea, a CPAP machine constantly pushes air into a person’s nose through a mask or apparatus strapped to the face. The airflow keeps the uvula, soft palate and tongue from obstructing the airway, allowing a person to breathe better and improve sleep. It also reduces snoring.
Medical providers program the machine with the correct pressure setting for the patient, and the pressure can be adjusted if needed. It may take a while to get used to sleeping with a CPAP.
Choosing the right CPAP machine is important. Patients have to use the device every time they sleep, including during naps, in order for it to work properly.
Side Effects of Philips CPAP Devices
Common Philips CPAP side effects may include dry eye, respiratory infections, skin irritation and nasal inflammation. Many patients cite discomfort from improper air pressure and the mask itself. Recalled Philips CPAP machines may cause more serious problems, including cancer.
Users of continuous positive airway pressure machines may experience common side effects from time to time, even after the initial adjustment period.
- Aerophagia
- Abdominal discomfort, acid reflux or flatulence can be caused by swallowing pressurized air.
- Claustrophobia
- Mask wear may trigger a patient’s fear of being in small or enclosed spaces.
- Discomfort
- Mask wear may be uncomfortable.
- Dry Eye
- CPAP-related dry eye is typically caused by air passing through the nose ducts, air leakage or bacteria trapped by the mask.
- Ear Pressure/Pain
- Middle ear pressure may be caused by breathing through CPAP machines.
- Facial Hair Growth Issues
- Children may experience reduced facial hair growth after prolonged CPAP usage.
- Insomnia
- Sleep issues can be caused by CPAP machine noise or the adjustment period.
- Nasal Inflammation or Dryness
- Nasal discomfort from continuous pressurized airflow may occur.
- Respiratory Infections
- These infections are often caused by bacteria in the mask or air pathway.
- Skin Irritation
- CPAP users may develop rashes, sores or marks due to a mask’s composition or fit. Skin irritation can become serious, and, in susceptible people, skin infection can develop.
- Sleep Apnea
- If pressure is inadequate due to air leaks, it will not reduce obstruction properly, and apnea can still occur.
- Tooth Shifting or Chipping
- Dental issues may result from tight chin straps or tongue position.
Talk to your physician about any concerns you have about CPAP side effects. Pressure adjustments or using a different mask may alleviate your symptoms. You may also ask your doctor about alternatives to CPAP.
Serious Side Effects of Philips CPAP Machines
Potential long-term side effects from recalled Philips CPAP machines include respiratory problems, asthma and cancer risks.
Several people have filed Philips CPAP lawsuits over their recalled devices, claiming that the company produced a defective product and failed to warn users of the potential risks.
- Abdominal cancers
- Asthma
- Hematopoietic cancer
- Liver cancer
- Lung cancer
- Mammary gland cancer
- Melanoma
- Organ damage
- Pancreatic cancer
- Prostate cancer
- Rectal cancer
- Respiratory issues
Philips identified several chemical particles and gases that users may have ingested by inhaling degrading foam while using the recalled CPAP machines. Ingested or inhaled PE-PUR foam particles or chemical gases may lead to carcinogenic, toxic or respiratory side effects.
- Dimethyl Diazine
- Associated with intestinal diseases and colorectal neoplasms, according to the National Institutes of Health (NIH).
- Phenol 2,6-bis(1,1-dimethylethyl)-4-(1-methylpropyl)
- Exposure may cause irritation to the eyes, nose, skin, throat and nervous system. Symptoms include weight loss, weakness, exhaustion, muscle aches, pain, liver and kidney damage, skin burns, tremors, convulsions and twitching
- Diethylene Glycol
- Can cause abdominal pain, diarrhea, dizziness, drowsiness, confusion, nausea, vomiting and unconsciousness; associated with acidosis, kidney diseases, acute kidney necrosis and liver injury
- Toluenediamine
- Can irritate eyes, skin, nose and throat or cause dermatitis; ataxia, tachycardia, nausea, vomiting, convulsions, respiratory depression, methemoglobinemia, cyanosis, headache, weakness, exhaustion, dizziness, bluish skin, liver injury and some types of cancers
Other Short-Term Side Effects of Philips CPAP Machines
Due to the effects of recalled Philips CPAP machines, patients may experience short-term symptoms such as difficulty breathing, cough and runny nose. Not all patients will have long-term health issues after inhaling particles from degraded foam.
- Allergic and skin reactions
- Airway inflammation (especially dangerous for people with lung disease or reduced breathing capacity)
- Chest pressure
- Cough
- Dizziness
- Headache
- Hypersensitivity reactions
- Irritation of the respiratory tract, eyes, nose and skin
- Nausea or vomiting
- Sinus infections
The severity of side effects may depend on how long you’ve used a recalled machine and your overall health. Consider informing your doctor of any symptoms, no matter how minor or occasional.
If you use a recalled CPAP machine for sleep apnea, talk to your physician about your options. Sometimes, not using the CPAP machine may be riskier than continuing to use it. You and your doctor can decide if the benefits of continuing to use that specific machine outweigh any risks.
Tips for Preventing CPAP Machine Side Effects
Tips for preventing CPAP side effects include keeping your machine clean and using it only as directed. If you experience side effects, it’s recommended that you speak with your doctor before you adjust or stop using your machine.
- Adjust your mask for a tight, comfortable fit. Try a different mask if needed.
- Clean the tube and mask daily and replace as required using manufacturer-recommended methods.
- Get used to the machine while awake to avoid claustrophobia. Start by holding the mask to your face, then work up to using it while sleeping. Different-sized masks may help.
- Gradually increase pressure if the machine has an adjustable feature. Consult with a doctor to adjust pressure settings if it doesn’t.
- Pressure sores can develop due to the mask. Using a nasal pad may help.
- Use a chinstrap to prevent dry mouth.
- Use humidification to reduce dryness. Always use sterile water to reduce the risk of infection.
The FDA advises against removing foam from recalled Philips CPAP machines, as this may lead to side effects or damage. Hot and humid environments, UV light and ozone cleaners can also degrade PE-PUR foam.
Philips Recalls CPAP Machines
On June 30, 2021, the U.S. Food and Drug Administration issued a Safety Communication about Philips’ voluntary recall of some of its BiPAP, CPAP and ventilator devices. Philips is the largest producer of CPAPs in the world, and as many as four million machines could be affected. People who can’t stop sleep apnea treatment may have to look for CPAP alternatives, such as oral implants or another brand of CPAP machine.
Philips recalled the devices after it discovered that polyester-based polyurethane (PE-PUR) sound abatement foam used to reduce sound and vibration in the devices may degrade and release particles and gases into the device’s air pathways. The CPAP maker said ingesting or inhaling foam particles or chemical gases could lead to toxic and carcinogenic effects. So far, no deaths have been reported.
Shortly after the recall was announced, lawyers began investigating Philips CPAP lawsuits on behalf of patients who used recalled devices and were diagnosed with cancer or other health issues, such as respiratory problems.
In September 2021, Philips began replacing PE-PUR foam in some machines with what they said was a safer silicone foam. But in November 2021, the FDA released a Philips CPAP recall update informing the public that the new silicone foam may also pose a potential health risk. The agency also released an inspection report that revealed that Philips knew as far back as 2015 that PE-PUR foam could degrade but didn’t inform the public.
In June 2022, Philips released some results of its PE-PUR testing. The company said the emissions of toxic chemicals were below established limits and are not anticipated to cause long-term health consequences. Philips also pointed to methods for cleaning a CPAP machine that involve devices that emit ozone gas to sanitize machines. These sanitizing devices are not currently FDA-approved. The FDA has yet to weigh in on these findings.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.