Viberzi Side Effects
Viberzi (eluxadoline) is a medication used to manage symptoms of irritable bowel syndrome (IBS) with diarrhea in adults. The most common side effects of the drug include nausea and stomach pain. The drug can also cause serious, less common side effects, such as severe constipation, pancreatitis and sphincter of Oddi spasm. It is not recommended for use in patients without a gallbladder.
Constipation, nausea and stomach pain are the most common side effects of Viberzi, according to the drug’s maker, Allergan.
Because the drug’s main ingredient, eluxadoline, can cause feelings of euphoria, Viberzi has abuse potential and may lead to drug dependence. Though infrequent, in clinical trials, patients who took the drug at normal dosages reported feelings of “euphoria” and “feeling drunk.”
The medication can also cause serious adverse reactions, such as pancreatitis and/or sphincter of Oddi spasm, that may require hospitalization. Patients without a gallbladder are at an increased risk of developing these serious, potentially fatal, adverse reactions. Use of Viberzi in patients without a gallbladder is not recommended.
A February 2022 study found that although Viberzi was at least as effective as antispasmodics in treating IBS pain, it has more reported side effects. Study authors recommended antispasmodics as the first line of treatment.
Constipation and Other Common Side Effects
Constipation was the most commonly reported adverse reaction in patients who took Viberzi during clinical trials. The drug’s safety information warns that constipation can become severe and may require hospitalization. It advises patients to stop taking the medication and tell their doctor if constipation lasts for more than four days.
In clinical trials, 8 percent of patients developed constipation when taking 100 mg of the medication twice a day. Seven percent of patients taking 75 mg twice daily experienced constipation. Participants taking a placebo developed the side effect at a rate of 2 percent. Rates of severe constipation were reported in less than 1 percent of patients taking 75 mg and 100 mg of Viberzi twice daily.
The majority of the constipation events took place within the first three months of treatment. About half happened within the first two weeks of therapy.
Since patients taking Viberzi are at a higher risk for constipation, continued use of other constipating drugs should be avoided. Patients with a history of chronic or severe constipation, or who have a known gastrointestinal obstruction, may be at risk for severe complications of bowel obstruction and should speak with their doctor before starting treatment.
Nausea and abdominal pain were each reported in 7 percent of clinical trial participants who took 100 mg twice daily. Eight percent of patients who took 75 mg twice daily reported nausea, while 6 percent of patients who took the same dosage reported abdominal pain. In participants given a placebo, 5 percent reported nausea, while 4 percent reported abdominal pain.
- Upper respiratory tract infections, including bronchitis
- Vomiting
- Common colds
- Dizziness
- Flatulence
- Rash
- Elevated liver enzymes
- Fatigue
- Viral gastroenteritis
These were reported in between 1 percent and 5 percent of clinical trial participants.
Viberzi can cause allergic reactions. Patients who experience signs or symptoms of an allergic reaction — such as hives, difficulty breathing, or swelling of the face, lips, tongue or throat —should seek emergency medical attention.
Serious Cases of Pancreatitis or Death
From when the FDA first approved Viberzi in May 2015 through February 2017, 120 reports of serious cases of pancreatitis or death associated with the drug were reported. In majority of these reports, patients did not have a gallbladder. Seventy-six patients were hospitalized, of which two died. These two patients did not have gallbladders.
The FDA issued a safety announcement in March 2017 warning people without gallbladders to avoid taking the drug. The federal agency warned that these patients have an increased risk of developing pancreatitis that could result in hospitalization or death.
Pancreatitis is an inflammation of the pancreas that causes severe pain in the upper abdomen. The pain may extend to the back. Some people experience nausea and vomiting. Other symptoms include fever, diarrhea, rapid pulse, weight loss, and oily or discolored stools.
Patients and their doctors reported that serious pancreatitis developed within a week of starting treatment with Viberzi. Some patients experienced symptoms after just one or two doses of the medicine.
A 2018 analysis of adverse event reports with Viberzi in the journal of Alimentary Pharmacology and Therapeutics noted that nearly 40 percent of pancreatitis cases developed within the first or second dose of the drug. About half of the reported cases occurred in patients without a gallbladder.
“Among the 68 patients who reported their gallbladder status, 56 of them did not have a gallbladder and received the currently recommended dosage of Viberzi.”
In the FDA reported case of fatal pancreatitis, the patient experienced severe abdominal pain, nausea and vomiting within one hour of taking a single dose of Viberzi. The patient died three days later.
A contraindication to use of the drug in patients without a gallbladder was added to the drug in November 2017. People who suffered pancreatitis while taking the medication are suing Allergan. Viberzi lawsuits say the company failed to warn of this potential risk.
Risk of Sphincter of Oddi Spasm
Some Viberzi users who developed pancreatitis also suffered sphincter of Oddi spasm. One person died from the condition, according to FDA data.
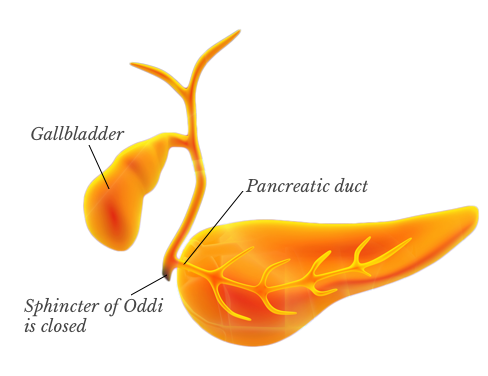
The sphincter of Oddi is a muscular valve surrounding the end of the common bile duct and pancreatic duct – also known as biliary ducts. The valve automatically opens during a meal to allow the juices from both ducts to flow into the small intestine.
Sphincter of Oddi spasm occurs when the valve is unable to open and close normally. This can cause a painful obstruction of the flow of bile, which can trigger pancreatitis.
Most of the reported cases in Viberzi users happened within one week of starting the drug. Some patients developed symptoms after one to two doses.
In the one fatal case reported to the FDA, the patient experienced severe abdominal pain and vomiting. The symptoms started soon after taking the first dose of Viberzi, according to the report. The patient did not have a gallbladder.
The drug’s safety information instructs patients who develop symptoms of the disorder to discontinue the drug immediately and seek medical attention. People with a history of sphincter of Oddi disease or obstructions of the bile ducts should not take the drug.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


